Vascular morphogenesis and remodeling in a human tumor xenograft: blood vessel formation and growth after ovariectomy and tumor implantation
- PMID: 11597997
- PMCID: PMC2752899
- DOI: 10.1161/hh2001.097872
Vascular morphogenesis and remodeling in a human tumor xenograft: blood vessel formation and growth after ovariectomy and tumor implantation
Abstract
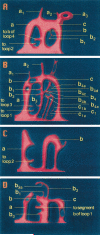
To determine mechanisms of blood vessel formation and growth in solid tumors, we used a model in which LS174T human colon adenocarcinomas are grown in the isolated ovarian pedicle of nude mice. Reconstruction of 3500 histological serial sections demonstrated that a new vascular network composed of venous-venous loops of varying sizes grows inside the tumor from the wall of the adjacent main vein. Loops elongate and remodel to establish complex loop systems. The mechanisms of loop formation and remodeling correspond to intussusceptive microvascular growth (IMG). In the tissue surrounding the tumor segmentation, another mechanism of IMG is prevalent in venous vessels. Comparison to vascular morphogenesis in the ovariectomized pedicle not only confirms the existence of corresponding mechanisms in both systems, but also reveals numerous sprouts that are superimposed onto loop systems and pathological deviations of loop formation, remodeling, and segmentation in the tumor. These pathological mechanisms interfere with vessel patency that likely cause heterogenous perfusion and hypoxia thus perpetuating angiogenesis. Blood vessel formation based on IMG was also detected in a large thrombus that completely occluded a part of an ovarian artery branch.
Figures




Comment in
-
Tubes, branches, and pillars: the many ways of forming a new vasculature.Circ Res. 2001 Oct 12;89(8):645-7. Circ Res. 2001. PMID: 11597985 No abstract available.
References
-
- Fülleborn F. Beiträge zur Entwicklung der Allantois der Vögel (dissertation). G. Schade; Berlin, Germany: 1895.
-
- Clark ER, Clark EL. Microscopic observations on the growth of blood capillaries in the living mammal. Am J Anat. 1939;64:251–299.
-
- Maximow A. Experimentelle Untersuchungen über die entzündliche Neubildung von Bindegewebe. In: Ziegler E, editor. Beiträge zur pathologischen Anatomie und zur allgemeinen Pathologie (suppl Heft 5) G. Fischer; Jena, Germany: 1902.
-
- Wakui S, Furusato M, Matsumoto I, Takai K, Ushigome S, Ishikawa E. Two- and three-dimensional ultrastructural observation of angiogenesis at a capillary sprout with front-to-front growth in a human granuloma. Microcirculation. 1987;2:797–798.
-
- Schoefl G. Studies on inflammation, III: growing capillaries: their structure and permeability. Virchows Arch Pathol Anat. 1963;337:97–141. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

