Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2
- PMID: 11598001
- PMCID: PMC125668
- DOI: 10.1093/emboj/20.20.5578
Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2
Abstract
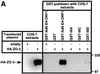
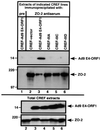
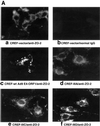
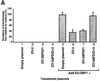
Adenovirus type 9 (Ad9) is distinct among human adenoviruses because it elicits solely mammary tumors in animals and its primary oncogenic determinant is the E4 region-encoded ORF1 (E4-ORF1) protein. We report here that the PDZ domain-containing protein ZO-2, which is a candidate tumor suppressor protein, is a cellular target for tumorigenic Ad9 E4-ORF1 but not for non-tumorigenic wild-type E4-ORF1 proteins encoded by adenovirus types 5 and 12. Complex formation was mediated by the C-terminal PDZ domain-binding motif of Ad9 E4- ORF1 and the first PDZ domain of ZO-2, and in cells this interaction resulted in aberrant sequestration of ZO-2 within the cytoplasm. Furthermore, transformation-defective Ad9 E4-ORF1 mutants exhibited impaired binding to and sequestration of ZO-2 in cells, and overexpression of wild-type ZO-2, but not mutant ZO-2 lacking the second and third PDZ domains, interfered with Ad9 E4-ORF1-induced focus formation. Our results suggest that the select capacity to complex with the candidate tumor suppressor protein ZO-2 is key to defining the unique transforming and tumorigenic properties of the Ad9 E4-ORF1 oncoprotein.
Figures




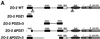
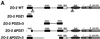



Similar articles
-
Several E4 region functions influence mammary tumorigenesis by human adenovirus type 9.J Virol. 2001 Jan;75(2):557-68. doi: 10.1128/JVI.75.2.557-568.2001. J Virol. 2001. PMID: 11134268 Free PMC article.
-
Adenovirus type 9 E4 open reading frame 1 encodes a transforming protein required for the production of mammary tumors in rats.J Virol. 1994 Jun;68(6):3917-24. doi: 10.1128/JVI.68.6.3917-3924.1994. J Virol. 1994. PMID: 8189528 Free PMC article.
-
Human adenovirus early region 4 open reading frame 1 genes encode growth-transforming proteins that may be distantly related to dUTP pyrophosphatase enzymes.J Virol. 1997 Mar;71(3):1857-70. doi: 10.1128/JVI.71.3.1857-1870.1997. J Virol. 1997. PMID: 9032316 Free PMC article.
-
Cell polarity proteins: common targets for tumorigenic human viruses.Oncogene. 2008 Nov 24;27(55):7031-46. doi: 10.1038/onc.2008.352. Oncogene. 2008. PMID: 19029943 Free PMC article. Review.
-
Adenovirus early E4 genes in viral oncogenesis.Oncogene. 2001 Nov 26;20(54):7847-54. doi: 10.1038/sj.onc.1204914. Oncogene. 2001. PMID: 11753667 Review.
Cited by
-
Polyubiquitination and SUMOylation Sites Regulate the Stability of ZO-2 Protein and the Sealing of Tight Junctions.Cells. 2022 Oct 19;11(20):3296. doi: 10.3390/cells11203296. Cells. 2022. PMID: 36291162 Free PMC article.
-
Roles of the PDZ domain-binding motif of the human papillomavirus type 16 E6 on the immortalization and differentiation of primary human foreskin keratinocytes.Virus Genes. 2014 Apr;48(2):224-32. doi: 10.1007/s11262-013-1017-9. Epub 2013 Nov 29. Virus Genes. 2014. PMID: 24293186
-
Altered expression of ZO-1 and ZO-2 in Sertoli cells and loss of blood-testis barrier integrity in testicular carcinoma in situ.Neoplasia. 2006 Dec;8(12):1019-27. doi: 10.1593/neo.06559. Neoplasia. 2006. PMID: 17217619 Free PMC article.
-
The human adenovirus E4-ORF1 protein subverts discs large 1 to mediate membrane recruitment and dysregulation of phosphatidylinositol 3-kinase.PLoS Pathog. 2014 May 1;10(5):e1004102. doi: 10.1371/journal.ppat.1004102. eCollection 2014 May. PLoS Pathog. 2014. PMID: 24788832 Free PMC article.
-
A new crucial protein interaction element that targets the adenovirus E4-ORF1 oncoprotein to membrane vesicles.J Virol. 2007 May;81(9):4787-97. doi: 10.1128/JVI.02855-06. Epub 2007 Feb 21. J Virol. 2007. PMID: 17314165 Free PMC article.
References
-
- Beatch M., Jesaitis,L.A., Gallin,W.J., Goodenough,D.A. and Stevenson,B.R. (1996) The tight junction protein ZO-2 contains three PDZ (PSD-95/Discs-Large/ZO-1) domains and an alternatively spliced region. J. Biol. Chem., 271, 25723–25726. - PubMed
-
- Ben-Ze’ev A. (1997) Cytoskeletal and adhesion proteins as tumor suppressors. Curr. Opin. Cell Biol., 9, 99–108. - PubMed
-
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248–254. - PubMed
-
- Chlenski A., Ketels,K.V., Engeriser,J.L., Talamonti,M.S., Tsao,M.S., Koutnikova,H., Oyasu,R. and Scarpelli,D.G. (1999a) ZO-2 gene alternative promoters in normal and neoplastic human pancreatic duct cells. Int. J. Cancer, 83, 349–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

