Administration of superantigens protects mice from lethal Listeria monocytogenes infection by enhancing cytotoxic T cells
- PMID: 11598032
- PMCID: PMC100037
- DOI: 10.1128/IAI.69.11.6633-6642.2001
Administration of superantigens protects mice from lethal Listeria monocytogenes infection by enhancing cytotoxic T cells
Abstract
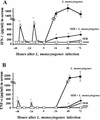
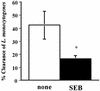
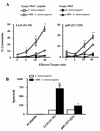
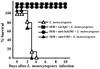
Superantigens stimulate T-cell-receptor Vbeta-selective T-cell proliferation accompanying the release of cytokines, which may eventually protect the host from microbial infections. We investigated here whether superantigens can rescue the host from lethal bacterial infection. Mice were pretreated with Staphylococcus aureus enterotoxin B (SEB) 1 and 2 days before bacterial infection, and the mortality of infected mice was assessed. SEB pretreatment protected mice from lethal infection with Listeria monocytogenes but not from lethal infection with Streptococcus pyogenes. This enhanced protection was also observed upon pretreatment with recombinant streptococcal pyrogenic exotoxin A. Furthermore, L. monocytogenes-specific delayed-type hypersensitivity (DTH) due to type 1 helper T (Th1) cells and the cytotoxicity of CD8(+) T cells were significantly enhanced after SEB administration and bacterial infection. Depletion of either CD4(+) T cells or CD8(+) T cells in SEB-pretreated mice completely abolished this protection. This phenomenon was ascribed to the elimination of L. monocytogenes-specific CD8(+) cytotoxic T lymphocytes (CTL). It was found that CD4(+) T cells contributed to the induction of the CTL populations. Furthermore, SEB pretreatment of heat-killed L. monocytogenes-immunized mice enhanced the protection from challenge of L. monocytogenes. Taken together, these results indicated that administrations of superantigens protected mice from infection with L. monocytogenes, which was dependent on the enhanced L. monocytogenes-specific CTL activity in the presence of CD4(+) T cells, and superantigens exhibited adjuvant activity in the immunization against intracellular pathogens.
Figures

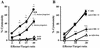


Similar articles
-
Enhancement of the Listeria monocytogenes p60-specific CD4 and CD8 T cell memory by nonpathogenic Listeria innocua.J Immunol. 1999 Apr 15;162(8):4781-9. J Immunol. 1999. PMID: 10202020
-
Stimulation of tumor-draining lymph node cells with superantigenic staphylococcal toxins leads to the generation of tumor-specific effector T cells.J Immunol. 1994 Feb 1;152(3):1277-88. J Immunol. 1994. PMID: 8301131
-
Systemic immunization induces protective CD4+ and CD8+ T cell-mediated immune responses in murine Listeria monocytogenes meningoencephalitis.Eur J Immunol. 1995 Aug;25(8):2384-91. doi: 10.1002/eji.1830250839. Eur J Immunol. 1995. PMID: 7664800
-
Listeria monocytogenes: a live vector able to deliver heterologous protein within the cytosol and to drive a CD8 dependent T cell response.Biologicals. 1995 Jun;23(2):135-43. doi: 10.1006/biol.1995.0024. Biologicals. 1995. PMID: 7546656 Review.
-
Superantigen as a modifying factor in HIV infection.Pediatr AIDS HIV Infect. 1996 Jun;7(3):143-54. Pediatr AIDS HIV Infect. 1996. PMID: 11361581 Review.
Cited by
-
Incidence and Effects of Acquisition of the Phage-Encoded ssa Superantigen Gene in Invasive Group A Streptococcus.Front Microbiol. 2021 Jun 4;12:685343. doi: 10.3389/fmicb.2021.685343. eCollection 2021. Front Microbiol. 2021. PMID: 34149675 Free PMC article.
-
Dietary plasma protein affects the immune response of weaned rats challenged with S. aureus Superantigen B.J Nutr. 2004 Oct;134(10):2667-72. doi: 10.1093/jn/134.10.2667. J Nutr. 2004. PMID: 15465764 Free PMC article.
-
The Streptococcus pyogenes capsule is required for adhesion of bacteria to virus-infected alveolar epithelial cells and lethal bacterial-viral superinfection.Infect Immun. 2004 Oct;72(10):6068-75. doi: 10.1128/IAI.72.10.6068-6075.2004. Infect Immun. 2004. PMID: 15385511 Free PMC article.
-
Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice.J Virol. 2003 Apr;77(7):4104-12. doi: 10.1128/jvi.77.7.4104-4112.2003. J Virol. 2003. PMID: 12634369 Free PMC article.
-
Nucleocapsid of rabies virus improve immune response of an inactivated avian influenza vaccine.Vet Res Commun. 2009 Oct;33(7):589-95. doi: 10.1007/s11259-009-9206-7. Epub 2009 Jan 31. Vet Res Commun. 2009. PMID: 19184631
References
-
- Bancroft G J, Sheehan K C F, Schreiber R D, Unanue E R. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–130. - PubMed
-
- Blackman M A, Woodland D L. In vivo effects of superantigens. Life Sci. 1995;57:1717–1735. - PubMed
-
- Buller R M L, Holmes K L, Hügin A, Frederickson T N, Morse H C., III Induction of cytotoxic T-cell responses in vivo in the absence of CD4 helper cells. Nature. 1987;328:77–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

