Type III secretion-dependent cell cycle block caused in HeLa cells by enteropathogenic Escherichia coli O103
- PMID: 11598051
- PMCID: PMC100056
- DOI: 10.1128/IAI.69.11.6785-6795.2001
Type III secretion-dependent cell cycle block caused in HeLa cells by enteropathogenic Escherichia coli O103
Abstract
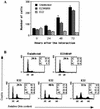
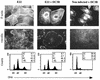
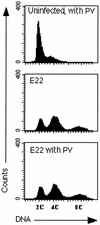
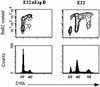
Rabbit enteropathogenic Escherichia coli (EPEC) O103 induces in HeLa cells an irreversible cytopathic effect characterized by the recruitment of focal adhesions, formation of stress fibers, and inhibition of cell proliferation. We have characterized the modalities of the proliferation arrest and investigated its underlying mechanisms. We found that HeLa cells that were exposed to the rabbit EPEC O103 strain E22 progressively accumulated at 4C DNA content and did not enter mitosis. A significant proportion of the cells were able to reinitiate DNA synthesis without division, leading to 8C DNA content. This cell cycle inhibition by E22 was abrogated in mutants lacking EspA, -B, and -D and was restored by transcomplementation. In contrast, intimin and Tir mutants retained the antiproliferative effect. The cell cycle arrest was not a direct consequence of the formation of stress fibers, since their disruption by toxins during exposure to E22 did not reverse the cell cycle inhibition. Likewise, the cell cycle arrest was not dependent on the early tyrosine dephosphorylation events triggered by E22 in the cells. Two key partner effectors controlling entry into mitosis were also investigated: cyclin B1 and the associated cyclin-dependent kinase 1 (Cdk1). Whereas cyclin B1 was not detectably affected in E22-exposed cells, Cdk1 was maintained in a tyrosine-phosphorylated inactive state and lost its affinity for p13(suc1)-agarose beads. This shows that Cdk1 is implicated in the G2/M arrest caused by EPEC strain E22.
Figures





References
-
- Assoian R K, Zhu X. Cell anchorage and the cytoskeleton as partners in growth factor dependent cell cycle progression. Curr Opin Cell Biol. 1997;9:93–98. - PubMed
-
- Barth H, Olenik C, Sehr P, Schmidt G, Aktories K, Meyer D K. Neosynthesis and activation of rho by Escherichia coli cytotoxic necrotizing factor (CNF1) reverse cytopathic effects of ADP-ribosylated Rho. J Biol Chem. 1999;274:27407–27414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

