Capsule production and growth phase influence binding of complement to Staphylococcus aureus
- PMID: 11598052
- PMCID: PMC100057
- DOI: 10.1128/IAI.69.11.6796-6803.2001
Capsule production and growth phase influence binding of complement to Staphylococcus aureus
Abstract
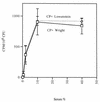
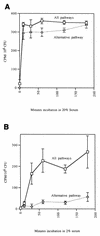
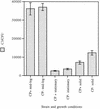
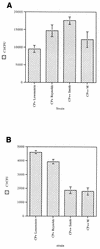
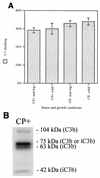
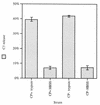
Complement-mediated opsonization of bacteria by C3 binding is an important component of the host innate immune system. Little information is available concerning the interaction between complement proteins and capsule type 5 and 8 Staphylococcus aureus strains, even though these isolates are responsible for approximately 70% of human staphylococcal infections. To investigate the importance of an intact complement pathway in an experimental staphylococcal infection, control and C3-depleted mice were challenged intravenously with 10(7) CFU of a serotype 5 S. aureus isolate. Whereas only 8% of the control mice succumbed to the infection, 64% of the complemented-depleted animals died. In vitro parameters of C3 binding to two heavily encapsulated (CP++) strains, three encapsulated (CP+) strains, and an isogenic capsule-negative (CP-) mutant were examined. The alternative pathway contributed 90% of C3 binding in 20% serum at 30 min, whereas it accounted for only 13% of C3 binding in 2% serum. Stationary-phase organisms bound only 10% as much C3 as mid-log-phase organisms; this was only in part due to capsule. When the S. aureus strains were cultivated on solid medium, the CP++ isolates bound 50% less C3 than CP+ strains; a CP+ strain bound 42% less C3 than the CP- mutant. Both C3b and iC3b fragments of C3 bound to S. aureus cells, and about one-third of the bound C3 was shed from the staphylococcal surface as iC3b, regardless of the CP phenotype of the strain. Thus, the phase of growth and presence of capsule are critical to opsonization.
Figures



Similar articles
-
Availability of complement bound to Staphylococcus aureus to interact with membrane complement receptors influences efficiency of phagocytosis.Infect Immun. 2003 Feb;71(2):656-62. doi: 10.1128/IAI.71.2.656-662.2003. Infect Immun. 2003. PMID: 12540542 Free PMC article.
-
Role of complement receptors 1 and 2 (CD35 and CD21), C3, C4, and C5 in survival by mice of Staphylococcus aureus bacteremia.J Lab Clin Med. 2004 Jun;143(6):358-65. doi: 10.1016/j.lab.2004.03.005. J Lab Clin Med. 2004. PMID: 15192652
-
Opsonization of encapsulated Staphylococcus aureus: the role of specific antibody and complement.J Immunol. 1982 Oct;129(4):1681-7. J Immunol. 1982. PMID: 7108223
-
Advances in understanding the structure, function, and mechanism of the SCIN and Efb families of Staphylococcal immune evasion proteins.Adv Exp Med Biol. 2012;946:113-33. doi: 10.1007/978-1-4614-0106-3_7. Adv Exp Med Biol. 2012. PMID: 21948365 Free PMC article. Review.
-
[The role of capsule in staphylococcal infection and immunity].Nihon Saikingaku Zasshi. 1996 Feb;51(2):601-11. doi: 10.3412/jsb.51.601. Nihon Saikingaku Zasshi. 1996. PMID: 8752383 Review. Japanese. No abstract available.
Cited by
-
Rapid and reliable identification of Staphylococcus aureus capsular serotypes by means of artificial neural network-assisted Fourier transform infrared spectroscopy.J Clin Microbiol. 2013 Jul;51(7):2261-6. doi: 10.1128/JCM.00581-13. Epub 2013 May 8. J Clin Microbiol. 2013. PMID: 23658268 Free PMC article.
-
Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus.Hum Vaccin Immunother. 2013 Mar;9(3):480-7. doi: 10.4161/hv.23223. Epub 2012 Dec 18. Hum Vaccin Immunother. 2013. PMID: 23249887 Free PMC article.
-
Classical complement activation on human erythrocytes in subjects with systemic lupus erythematosus and a history of autoimmune hemolytic anemia.Lupus. 2020 Sep;29(10):1179-1188. doi: 10.1177/0961203320936347. Epub 2020 Jul 12. Lupus. 2020. PMID: 32659155 Free PMC article.
-
The Staphylococcus aureus polysaccharide capsule and Efb-dependent fibrinogen shield act in concert to protect against phagocytosis.Microbiology (Reading). 2016 Jul;162(7):1185-1194. doi: 10.1099/mic.0.000293. Epub 2016 Apr 25. Microbiology (Reading). 2016. PMID: 27112346 Free PMC article.
-
Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model.Infect Immun. 2006 Apr;74(4):2145-53. doi: 10.1128/IAI.74.4.2145-2153.2006. Infect Immun. 2006. PMID: 16552044 Free PMC article.
References
-
- Ahern J M, Rosengard A M. Complement receptors. In: Volanakis J E, Frank M M, editors. The human complement system in health and disease. New York, N.Y: Marcel Dekker; 1998. pp. 167–202.
-
- Baddour L M, Lowrance C, Albus A, Lowrance J H, Anderson S K, Lee J C. Staphylococcus aureus microcapsule expression attenuates bacterial virulence in a rat model of experimental endocarditis. J Infect Dis. 1992;165:749–753. - PubMed
-
- Centers for Disease Control and Prevention. National Nosocomial Infection Surveillance System report: data summary from October 1986–April 1996. U.S. Atlanta, Ga: Department of Health and Human Services; 1996.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

