Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus
- PMID: 11598064
- PMCID: PMC100069
- DOI: 10.1128/IAI.69.11.6893-6901.2001
Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus
Abstract
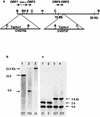
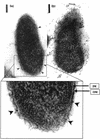
Virulence of Vibrio vulnificus correlates with changes in colony morphology that are indicative of a reversible phase variation for expression of capsular polysaccharide (CPS). Encapsulated variants are virulent with opaque colonies, whereas phase variants with reduced CPS expression are attenuated and are translucent. Using TnphoA mutagenesis, we identified a V. vulnificus CPS locus, which included an upstream ops element, a wza gene (wza(Vv)), and several open reading frames with homology to CPS biosynthetic genes. This genetic organization is characteristic of group 1 CPS operons. The wza gene product is required for transport of CPS to the cell surface in Escherichia coli. Polar transposon mutations in wza(Vv) eliminated expression of downstream biosynthetic genes, confirming operon structure. On the other hand, nonpolar inactivation of wza(Vv) was specific for CPS transport, did not alter CPS biosynthesis, and could be complemented in trans. Southern analysis of CPS phase variants revealed deletions or rearrangements at this locus. A survey of environmental isolates indicated a correlation between deletions in wza(Vv) and loss of virulent phenotype, suggesting a genetic mechanism for CPS phase variation. Full virulence in mice required surface expression of CPS and supported the essential role of capsule in the pathogenesis of V. vulnificus.
Figures




Similar articles
-
Structure, Function, and Regulation of the Essential Virulence Factor Capsular Polysaccharide of Vibrio vulnificus.Int J Mol Sci. 2020 May 5;21(9):3259. doi: 10.3390/ijms21093259. Int J Mol Sci. 2020. PMID: 32380667 Free PMC article. Review.
-
Genetic variation in the Vibrio vulnificus group 1 capsular polysaccharide operon.J Bacteriol. 2006 Mar;188(5):1987-98. doi: 10.1128/JB.188.5.1987-1998.2006. J Bacteriol. 2006. PMID: 16484211 Free PMC article.
-
Phase variation, capsular polysaccharide, pilus and flagella contribute to uptake of Vibrio vulnificus by the Eastern oyster (Crassostrea virginica).Environ Microbiol. 2009 Aug;11(8):1934-44. doi: 10.1111/j.1462-2920.2009.01916.x. Environ Microbiol. 2009. PMID: 19689704
-
Differential expression of Vibrio vulnificus capsular polysaccharide.Infect Immun. 1999 May;67(5):2250-7. doi: 10.1128/IAI.67.5.2250-2257.1999. Infect Immun. 1999. PMID: 10225881 Free PMC article.
-
Characterization of clinical and environmental types of Vibrio vulnificus isolates from Louisiana oysters.Foodborne Pathog Dis. 2009 Dec;6(10):1251-8. doi: 10.1089/fpd.2009.0343. Foodborne Pathog Dis. 2009. PMID: 19743928
Cited by
-
Structure, Function, and Regulation of the Essential Virulence Factor Capsular Polysaccharide of Vibrio vulnificus.Int J Mol Sci. 2020 May 5;21(9):3259. doi: 10.3390/ijms21093259. Int J Mol Sci. 2020. PMID: 32380667 Free PMC article. Review.
-
Characterization of self-generated variants in Pseudoalteromonas lipolytica biofilm with increased antifouling activities.Appl Microbiol Biotechnol. 2015 Dec;99(23):10127-39. doi: 10.1007/s00253-015-6865-x. Epub 2015 Aug 12. Appl Microbiol Biotechnol. 2015. PMID: 26264135 Free PMC article.
-
Vibrio vulnificus damages macrophages during the early phase of infection.Infect Immun. 2007 Sep;75(9):4592-6. doi: 10.1128/IAI.00481-07. Epub 2007 Jun 25. Infect Immun. 2007. PMID: 17591793 Free PMC article.
-
Sewage Promotes Vibrio vulnificus Growth and Alters Gene Transcription in Vibrio vulnificus CMCP6.Microbiol Spectr. 2022 Feb 23;10(1):e0191321. doi: 10.1128/spectrum.01913-21. Epub 2022 Feb 16. Microbiol Spectr. 2022. PMID: 35171011 Free PMC article.
-
Vibrio vulnificus biotype 2 serovar E gne but not galE is essential for lipopolysaccharide biosynthesis and virulence.Infect Immun. 2008 Apr;76(4):1628-38. doi: 10.1128/IAI.01393-07. Epub 2008 Jan 28. Infect Immun. 2008. PMID: 18227162 Free PMC article.
References
-
- Bayer M, Bayer M H. Biophysical and structural aspects of the bacterial capsule. ASM News. 1994;60:192–198.
-
- Blake P A, Merson M H, Weaver R E, Hollis D G, Heublein P C. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N Engl J Med. 1979;300:1–5. - PubMed
-
- Brown R C, Taylor R K. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol Microbiol. 1995;16:425–439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

