Generation and surface localization of intact M protein in Streptococcus pyogenes are dependent on sagA
- PMID: 11598078
- PMCID: PMC100083
- DOI: 10.1128/IAI.69.11.7029-7038.2001
Generation and surface localization of intact M protein in Streptococcus pyogenes are dependent on sagA
Abstract
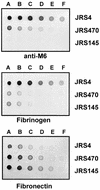
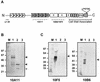
The M protein is an important surface-located virulence factor of Streptococcus pyogenes, the group A streptococcus (GAS). Expression of M protein is primarily controlled by Mga, a transcriptional activator protein. A recent report suggested that the sag locus, which includes nine genes necessary and sufficient for production of streptolysin S, another GAS virulence factor, is also needed for transcription of emm, encoding the M protein (Z. Li, D. D. Sledjeski, B. Kreikemeyer, A. Podbielski, and M. D. Boyle, J. Bacteriol. 181:6019-6027, 1999). To investigate this in more detail, we constructed an insertion-deletion mutation in sagA, the first gene in the sag locus, in the M6 strain JRS4. The resulting strain, JRS470, produced no detectable streptolysin S and showed a drastic reduction in cell surface-associated M protein, as measured by cell aggregation and Western blot analysis. However, transcription of the emm gene was unaffected by the sagA mutation. Detailed analysis with monoclonal antibodies and an antipeptide antibody showed that the M protein in the sagA mutant strain was truncated so that it lacks the C-repeat region and the C-terminal domain required for anchoring it to the cell surface. This truncated M protein was largely found, as expected, in the culture supernatant. Lack of surface-located M protein made the sagA mutant strain susceptible to phagocytosis. Thus, although sagA does not affect transcription of the M6 protein gene, it is needed for the surface localization of this important virulence factor.
Figures
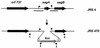

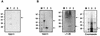
References
-
- Akesson P, Sjoholm A G, Bjorck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996;271:1081–1088. - PubMed
-
- Berge A, Bjorck L. Streptococcal cysteine proteinase releases biologically active fragments of streptococcal surface proteins. J Biol Chem. 1995;270:9862–9867. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

