Characterization of a di-leucine-based signal in the cytoplasmic tail of the nucleotide-pyrophosphatase NPP1 that mediates basolateral targeting but not endocytosis
- PMID: 11598187
- PMCID: PMC60151
- DOI: 10.1091/mbc.12.10.3004
Characterization of a di-leucine-based signal in the cytoplasmic tail of the nucleotide-pyrophosphatase NPP1 that mediates basolateral targeting but not endocytosis
Abstract
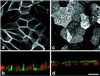
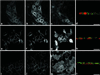
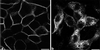
Enzymes of the nucleotide pyrophosphatase/phosphodiesterase (NPPase) family are expressed at opposite surfaces in polarized epithelial cells. We investigated the targeting signal of NPP1, which is exclusively expressed at the basolateral surface. Full-length NPP1 and different constructs and mutants were transfected into the polarized MDCK cell line. Expression of the proteins was analyzed by confocal microscopy and surface biotinylation. The basolateral signal of NPP1 was identified as a di-leucine motif located in the cytoplasmic tail. Mutation of either or both leucines largely redirected NPP1 to the apical surface. Furthermore, addition of the conserved sequence AAASLLAP redirected the apical nucleotide pyrophosphatase/phosphodiesterase NPP3 to the basolateral surface. Full-length NPP1 was not significantly internalized. However, when the cytoplasmic tail was deleted upstream the di-leucine motif or when the six upstream flanking amino acids were deleted, the protein was mainly found intracellularly. Endocytosis experiments indicated that these mutants were endocytosed from the basolateral surface. These results identify the basolateral signal of NPP1 as a short sequence including a di-leucine motif that is dominant over apical determinants and point to the importance of surrounding amino acids in determining whether the signal will function as a basolateral signal only or as an endocytotic signal as well.
Figures







References
-
- Ali SA, Steinkasserer A. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. Biotechniques. 1995;18:746–750. - PubMed
-
- Aroeti B, Okhrimenko H, Reich V, Orzech E. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim Biophys Acta. 1998;1376:57–90. - PubMed
-
- Belli SI, Goding JW. Biochemical characterization of human PC-1, an enzyme possessing alkaline phosphodiesterase I and nucleotide pyrophosphatase activities. Eur J Biochem. 1994;226:433–443. - PubMed
-
- Bollen M, Gijsbers R, Ceulemans H, Stalmans W, Stefan C. Nucleotide pyrophosphatases/phosphodiesterases on the move. Crit Rev Biochem Mol Biol. 2000;35:393–432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

