Lumenal endosomal and Golgi-retrieval determinants involved in pH-sensitive targeting of an early Golgi protein
- PMID: 11598199
- PMCID: PMC60163
- DOI: 10.1091/mbc.12.10.3152
Lumenal endosomal and Golgi-retrieval determinants involved in pH-sensitive targeting of an early Golgi protein
Abstract
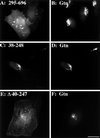
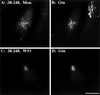
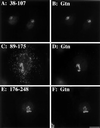
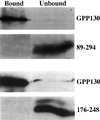
Despite the potential importance of retrieval-based targeting, few Golgi cisternae-localized proteins have been demonstrated to be targeted by retrieval, and the putative retrieval signals remain unknown. Golgi phosphoprotein of 130 kDa (GPP130) is a cis-Golgi protein that allows assay of retrieval-based targeting because it redistributes to endosomes upon treatment with agents that disrupt lumenal pH, and it undergoes endosome-to-Golgi retrieval upon drug removal. Analysis of chimeric molecules containing domains from GPP130 and the plasma membrane protein dipeptidylpeptidase IV indicated that GPP130 targeting information is contained entirely within its lumenal domain. Dissection of the lumenal domain indicated that a predicted coiled-coil stem domain adjacent to the transmembrane domain was both required and sufficient for pH-sensitive Golgi localization and endosome-to-Golgi retrieval. Further dissection of this stem domain revealed two noncontiguous stretches that each conferred Golgi localization separated by a stretch that conferred endosomal targeting. Importantly, in the absence of the endosomal determinant the Golgi targeting of constructs containing either or both of the Golgi determinants became insensitive to pH disruption by monensin. Because monensin blocks endosome-to-Golgi transport, the finding that the endosomal determinant confers monensin sensitivity suggests that the endosomal determinant causes GPP130 to traffic to endosomes from which it is normally retrieved. Thus, our observations identify Golgi and endosomal targeting determinants within a lumenal predicted coiled-coil domain that appear to act coordinately to mediate retrieval-based targeting of GPP130.
Figures





References
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York, NY: John Wiley & Sons; 1995.
-
- Bonfanti L, Mironov AA, Jr, Martinez-Menarguez JA, Martella O, Fusella A, Baldassarre M, Buccione R, Geuze HJ, Mironov AA, Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. - PubMed
-
- Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

