The cytoplasmic domain of the integrin alpha9 subunit requires the adaptor protein paxillin to inhibit cell spreading but promotes cell migration in a paxillin-independent manner
- PMID: 11598204
- PMCID: PMC60168
- DOI: 10.1091/mbc.12.10.3214
The cytoplasmic domain of the integrin alpha9 subunit requires the adaptor protein paxillin to inhibit cell spreading but promotes cell migration in a paxillin-independent manner
Abstract
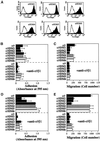
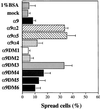
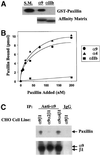
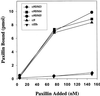
The integrin alpha9 subunit forms a single heterodimer, alpha9beta1. The alpha9 subunit is most closely related to the alpha4 subunit, and like alpha4 integrins, alpha9beta1 plays an important role in leukocyte migration. The alpha4 cytoplasmic domain preferentially enhances cell migration and inhibits cell spreading, effects that depend on interaction with the adaptor protein, paxillin. To determine whether the alpha9 cytoplasmic domain has similar effects, a series of chimeric and deleted alpha9 constructs were expressed in Chinese hamster ovary cells and tested for their effects on migration and spreading on an alpha9beta1-specific ligand. Like alpha4, the alpha9 cytoplasmic domain enhanced cell migration and inhibited cell spreading. Paxillin also specifically bound the alpha9 cytoplasmic domain and to a similar level as alpha4. In paxillin(-/-) cells, alpha9 failed to inhibit cell spreading as expected but surprisingly still enhanced cell migration. Further, mutations that abolished the alpha9-paxillin interaction prevented alpha9 from inhibiting cell spreading but had no effect on alpha9-dependent cell migration. These findings suggest that the mechanisms by which the cytoplasmic domains of integrin alpha subunits enhance migration and inhibit cell spreading are distinct and that the alpha9 and alpha4 cytoplasmic domains, despite sequence and functional similarities, enhance cell migration by different intracellular signaling pathways.
Figures





References
-
- Chan BM, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992;68:1051–1060. - PubMed
-
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. - PubMed
-
- Eto K, Puzon-McLaughlin W, Sheppard D, Sehara-Fujisawa A, Zhang XP, Takada Y. RGD-independent binding of integrin alpha9beta1 to the ADAM-12 and -15 disintegrin domains mediates cell-cell interaction. J Biol Chem. 2000;275:34922–34930. - PubMed
-
- Horwitz AR, Parsons JT. Cell migration: movin' on [comment] Science. 1999;286:1102–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

