Efficacies of moxifloxacin, ciprofloxacin, and vancomycin against experimental endocarditis due to methicillin-resistant Staphylococcus aureus expressing various degrees of ciprofloxacin resistance
- PMID: 11600359
- PMCID: PMC90785
- DOI: 10.1128/AAC.45.11.3076-3083.2001
Efficacies of moxifloxacin, ciprofloxacin, and vancomycin against experimental endocarditis due to methicillin-resistant Staphylococcus aureus expressing various degrees of ciprofloxacin resistance
Abstract
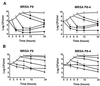
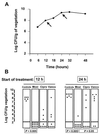
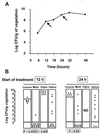
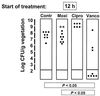
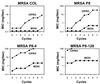
The new 8-methoxyquinolone moxifloxacin was tested against two ciprofloxacin-susceptible Staphylococcus aureus strains (strains P8 and COL) and two ciprofloxacin-resistant derivatives of strain P8 carrying a single grlA mutation (strain P8-4) and double grlA and gyrA mutations (strain P8-128). All strains were resistant to methicillin. The MICs of ciprofloxacin and moxifloxacin were 0.5 and 0.125 mg/liter, respectively, for P8; 0.25 and 0.125 mg/liter, respectively, for COL; 8 and 0.25 mg/liter, respectively, for P8-4; and >or=128 and 2 mg/liter, respectively, for P8-128. In vitro, the rate of spontaneous resistance of P8 and COL was 10(-7) on agar plates containing ciprofloxacin at two times the MIC, whereas it was <or=10(-10) on agar plates containing moxifloxacin at two times the MIC. Rats with experimental aortic endocarditis were treated with doses of drugs that simulate the kinetics in humans: moxifloxacin, 400 mg orally once a day; ciprofloxacin, 750 mg orally twice a day; or vancomycin, 1 g intravenously twice a day. Treatment was started either 12 or 24 h after infection and lasted for 3 days. Moxifloxacin treatment resulted in culture-negative vegetations in a total of 20 of 21 (95%) rats infected with P8, 10 of 11 (91%) rats infected with COL, and 19 of 24 (79%) rats infected with P8-4 (P < 0.05 compared to the results for the controls). In contrast, ciprofloxacin treatment sterilized zero of nine (0%) vegetations infected with first-level resistant mutant P8-4. Vancomycin sterilized only 8 of 15 (53%), 6 of 11 (54%), and 12 of 23 (52%) of the vegetations, respectively. No moxifloxacin-resistant derivative emerged among these organisms. However, moxifloxacin treatment of highly ciprofloxacin-resistant mutant P8-128 failed and selected for variants for which the MIC increased two times in 2 of 10 animals. Thus, while oral moxifloxacin might deserve consideration as treatment for staphylococcal infections in humans, caution related to its use against strains for which MICs are borderline is warranted.
Figures
References
-
- Andes D R, Craig W A. Pharmacodynamics of fluoroquinolones in experimental models of endocarditis. Clin Infect Dis. 1998;27:47–50. - PubMed
-
- Bauernfeind A. Comparison of the antibacterial activities of the quinolones Bay 12-8039, gatifloxacin (AM 1155), trovafloxacin, clinafloxacin, levofloxacin and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. - PubMed
-
- Brighty K E, Gootz T D. The chemistry and biological profile and trovafloxacin. J Antimicrob Chemother. 1997;39(Suppl. B):1–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

