Quinolone resistance mutations in Streptococcus pneumoniae GyrA and ParC proteins: mechanistic insights into quinolone action from enzymatic analysis, intracellular levels, and phenotypes of wild-type and mutant proteins
- PMID: 11600369
- PMCID: PMC90795
- DOI: 10.1128/AAC.45.11.3140-3147.2001
Quinolone resistance mutations in Streptococcus pneumoniae GyrA and ParC proteins: mechanistic insights into quinolone action from enzymatic analysis, intracellular levels, and phenotypes of wild-type and mutant proteins
Abstract
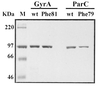
Mutations in DNA gyrase and/or topoisomerase IV genes are frequently encountered in quinolone-resistant mutants of Streptococcus pneumoniae. To investigate the mechanism of their effects at the molecular and cellular levels, we have used an Escherichia coli system to overexpress S. pneumoniae gyrase gyrA and topoisomerase IV parC genes encoding respective Ser81Phe and Ser79Phe mutations, two changes widely associated with quinolone resistance. Nickel chelate chromatography yielded highly purified mutant His-tagged proteins that, in the presence of the corresponding GyrB and ParE subunits, reconstituted gyrase and topoisomerase IV complexes with wild-type specific activities. In enzyme inhibition or DNA cleavage assays, these mutant enzyme complexes were at least 8- to 16-fold less responsive to both sparfloxacin and ciprofloxacin. The ciprofloxacin-resistant (Cip(r)) phenotype was silent in a sparfloxacin-resistant (Spx(r)) S. pneumoniae gyrA (Ser81Phe) strain expressing a demonstrably wild-type topoisomerase IV, whereas Spx(r) was silent in a Cip(r) parC (Ser79Phe) strain. These epistatic effects provide strong support for a model in which quinolones kill S. pneumoniae by acting not as enzyme inhibitors but as cellular poisons, with sparfloxacin killing preferentially through gyrase and ciprofloxacin through topoisomerase IV. By immunoblotting using subunit-specific antisera, intracellular GyrA/GyrB levels were a modest threefold higher than those of ParC/ParE, most likely insufficient to allow selective drug action by counterbalancing the 20- to 40-fold preference for cleavable-complex formation through topoisomerase IV observed in vitro. To reconcile these results, we suggest that drug-dependent differences in the efficiency by which ternary complexes are formed, processed, or repaired in S. pneumoniae may be key factors determining the killing pathway.
Figures




Similar articles
-
Ciprofloxacin dimers target gyrase in Streptococcus pneumoniae.Antimicrob Agents Chemother. 2004 Jun;48(6):2108-15. doi: 10.1128/AAC.48.6.2108-2115.2004. Antimicrob Agents Chemother. 2004. PMID: 15155208 Free PMC article.
-
Mutant prevention concentrations for single-step fluoroquinolone-resistant mutants of wild-type, efflux-positive, or ParC or GyrA mutation-containing Streptococcus pneumoniae isolates.Antimicrob Agents Chemother. 2004 Oct;48(10):3954-8. doi: 10.1128/AAC.48.10.3954-3958.2004. Antimicrob Agents Chemother. 2004. PMID: 15388458 Free PMC article.
-
In vivo pharmacodynamic efficacy of gatifloxacin against Streptococcus pneumoniae in an experimental model of pneumonia: impact of the low levels of fluoroquinolone resistance on the enrichment of resistant mutants.J Antimicrob Chemother. 2004 Sep;54(3):640-7. doi: 10.1093/jac/dkh393. Epub 2004 Aug 18. J Antimicrob Chemother. 2004. PMID: 15317743
-
Dual activity of fluoroquinolones against Streptococcus pneumoniae: the facts behind the claims.J Antimicrob Chemother. 2002 Jun;49(6):893-5. doi: 10.1093/jac/dkf047. J Antimicrob Chemother. 2002. PMID: 12039880 Review. No abstract available.
-
Quinolone mode of action.Drugs. 1995;49 Suppl 2:10-5. doi: 10.2165/00003495-199500492-00004. Drugs. 1995. PMID: 8549276 Review.
Cited by
-
Mutagenesis in the alpha3alpha4 GyrA helix and in the Toprim domain of GyrB refines the contribution of Mycobacterium tuberculosis DNA gyrase to intrinsic resistance to quinolones.Antimicrob Agents Chemother. 2008 Aug;52(8):2909-14. doi: 10.1128/AAC.01380-07. Epub 2008 Apr 21. Antimicrob Agents Chemother. 2008. PMID: 18426901 Free PMC article.
-
Clerocidin selectively modifies the gyrase-DNA gate to induce irreversible and reversible DNA damage.Nucleic Acids Res. 2008 Oct;36(17):5516-29. doi: 10.1093/nar/gkn539. Epub 2008 Aug 22. Nucleic Acids Res. 2008. PMID: 18723572 Free PMC article.
-
ATP-bound conformation of topoisomerase IV: a possible target for quinolones in Streptococcus pneumoniae.J Bacteriol. 2003 Oct;185(20):6137-46. doi: 10.1128/JB.185.20.6137-6146.2003. J Bacteriol. 2003. PMID: 14526026 Free PMC article.
-
Mechanism of quinolone action and resistance.Biochemistry. 2014 Mar 18;53(10):1565-74. doi: 10.1021/bi5000564. Epub 2014 Mar 7. Biochemistry. 2014. PMID: 24576155 Free PMC article. Review.
-
Small-colony mutants of Staphylococcus aureus allow selection of gyrase-mediated resistance to dual-target fluoroquinolones.Antimicrob Agents Chemother. 2002 Aug;46(8):2498-506. doi: 10.1128/AAC.46.8.2498-2506.2002. Antimicrob Agents Chemother. 2002. PMID: 12121924 Free PMC article.
References
-
- Adams D E, Shekhtman E M, Zechiedrich E L, Schmid M B, Cozzarelli N R. The role of topoisomerase IV in partitioning DNA replicons and the structure of catenated intermediates in DNA replication. Cell. 1992;71:277–288. - PubMed
-
- Alovero F L, Pan X-S, Morris J E, Manzo R H, Fisher L M. Engineering the specificity of antibacterial fluoroquinolones: benzenesulfonamide modifications at C-7 of ciprofloxacin change its primary target in Streptococcus pneumoniae from topoisomerase IV to gyrase. Antimicrob Agents Chemother. 2000;44:320–325. - PMC - PubMed
-
- Austin C A, Marsh K L, Wasserman R A, Willmore E, Sayer P J, Wang J C, Fisher L M. Expression, domain structure and enzymatic properties of an active recombinant human topoisomerase IIβ. J Biol Chem. 1995;276:15739–15746. - PubMed
-
- Bartlett J G, Grundy L M. Community-acquired pneumonia. New Engl J Med. 1995;333:1618–1624. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

