Attenuation of G protein-mediated inhibition of N-type calcium currents by expression of caveolins in mammalian NG108-15 cells
- PMID: 11600672
- PMCID: PMC2278875
- DOI: 10.1111/j.1469-7793.2001.0361c.xd
Attenuation of G protein-mediated inhibition of N-type calcium currents by expression of caveolins in mammalian NG108-15 cells
Abstract
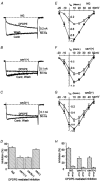
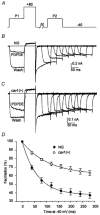
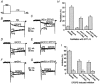
1. Caveolins are integral proteins of glycolipid/cholesterol-rich plasmalemmal caveolae domains, where, they may function as a plasma membrane scaffold onto which many classes of signalling molecules, including receptors and heterotrimeric G proteins, can assemble. To ascertain whether caveolins influence G protein-mediated signal transduction, we stably expressed caveolin-1 and -3 isoforms in the neuroblastoma x glioma NG108-15 hybrid cell line, lacking endogenous caveolins. Subsequently, using whole-cell voltage clamp methods, we examined whether the modulation of N-type voltage-gated Ca2+ channels by G(o) protein-coupled, delta-type opioid receptors might be affected by recombinant caveolin expression. 2. In transfected NG108-15 cells, caveolins localized at the plasma membrane and, upon subcellular fractionation on sucrose density gradients, they co-localized in Triton-resistant, low buoyancy fractions, with endogenous G(o) protein alpha-subunits. 3. The voltage-dependent inhibition of omega-conotoxin GVIA-sensitive Ba2+ currents following either activation of delta-opioid receptors by the agonist [o-pen2,o-pen5]-enkephalin (DPDPE), or direct stimulation of G proteins with guanosine 5'-O-(thiotriphosphate) (GTPgammaS) was significantly attenuated in caveolin-expressing cells. The kinetics of Ca2+ channel inhibition were also modified by caveolins. 4. Overall, these results suggest that caveolins may negatively affect G protein-dependent regulation of voltage-gated N-type Ca2+ channels, presumably by causing a reduction of the available pool of activated G proteins.
Figures




Similar articles
-
Caveolin-1 expression and membrane cholesterol content modulate N-type calcium channel activity in NG108-15 cells.Biophys J. 2005 Oct;89(4):2443-57. doi: 10.1529/biophysj.105.065623. Epub 2005 Jul 22. Biophys J. 2005. PMID: 16040758 Free PMC article.
-
Coupling of the cloned mu-opioid receptor with the omega-conotoxin-sensitive Ca2+ current in NG108-15 cells.J Neurochem. 1995 Sep;65(3):1403-6. doi: 10.1046/j.1471-4159.1995.65031403.x. J Neurochem. 1995. PMID: 7643119
-
Modulation of N-type calcium channel activity by G-proteins and protein kinase C.J Gen Physiol. 2000 Mar;115(3):277-86. doi: 10.1085/jgp.115.3.277. J Gen Physiol. 2000. PMID: 10694257 Free PMC article.
-
Receptor-mediated modulation of voltage-dependent Ca2+ channels via heterotrimeric G-proteins in neurons.Jpn J Pharmacol. 1999 Dec;81(4):324-31. doi: 10.1254/jjp.81.324. Jpn J Pharmacol. 1999. PMID: 10669036 Review.
-
Caveolin proteins and estrogen signaling in the brain.Mol Cell Endocrinol. 2008 Aug 13;290(1-2):8-13. doi: 10.1016/j.mce.2008.04.005. Epub 2008 Apr 22. Mol Cell Endocrinol. 2008. PMID: 18502030 Free PMC article. Review.
Cited by
-
Palmitic is the main fatty acid carried by lipids of detergent-resistant membrane fractions from neural and non-neural cells.Neurochem Res. 2002 Aug;27(7-8):729-34. doi: 10.1023/a:1020240520465. Neurochem Res. 2002. PMID: 12374207
-
Caveolin-1 expression and membrane cholesterol content modulate N-type calcium channel activity in NG108-15 cells.Biophys J. 2005 Oct;89(4):2443-57. doi: 10.1529/biophysj.105.065623. Epub 2005 Jul 22. Biophys J. 2005. PMID: 16040758 Free PMC article.
-
Molecular Pharmacology of δ-Opioid Receptors.Pharmacol Rev. 2016 Jul;68(3):631-700. doi: 10.1124/pr.114.008979. Pharmacol Rev. 2016. PMID: 27343248 Free PMC article. Review.
-
Talin-1 interaction network promotes hepatocellular carcinoma progression.Oncotarget. 2017 Feb 21;8(8):13003-13014. doi: 10.18632/oncotarget.14674. Oncotarget. 2017. PMID: 28099903 Free PMC article.
-
Cholesterol reduction by methyl-beta-cyclodextrin attenuates the delta opioid receptor-mediated signaling in neuronal cells but enhances it in non-neuronal cells.Biochem Pharmacol. 2007 Feb 15;73(4):534-49. doi: 10.1016/j.bcp.2006.10.032. Epub 2006 Nov 3. Biochem Pharmacol. 2007. PMID: 17141202 Free PMC article.
References
-
- Anderson RGW. The caveolae membrane system. Annual Review of Biochemistry. 1998;67:199–225. - PubMed
-
- Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. - PubMed
-
- De Waard M, Liu H, Walker D, Scott VE, Gurnett CA, Campbell KP. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

