Gradient of sodium current across the left ventricular wall of adult rat hearts
- PMID: 11600679
- PMCID: PMC2278873
- DOI: 10.1111/j.1469-7793.2001.0439c.xd
Gradient of sodium current across the left ventricular wall of adult rat hearts
Abstract
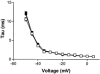
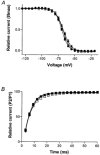
1. Gradients of ion channels across the left ventricular (LV) wall have been well characterized and it has been shown that disruption of such gradients leads to altered rates of repolarization across the wall, which is associated with the generation of arrhythmias. 2. We have hypothesized that a transmural gradient of I(Na) is present and have directly measured this current in adult rat myocytes isolated from both the epicardial and endocardial layers of the left ventricle. Currents were also recorded in right ventricular (RV) myocytes for comparison. 3. Peak inward I(Na) currents, at -30 mV, were -49.7 +/- 2.5 pA pF(-1) (n = 22), -32.9 +/- 3.2 pA pF(-1) (n = 16) and -49.7 +/- 3.7 pA pF(-1) (n = 24) for RV, LV epicardial and LV endocardial myocytes, respectively. No differences in the voltage dependence of inactivation, the voltage dependence of steady-state inactivation, or reactivation were reported. 4. Our results demonstrate that a gradient of sodium current density is present across the LV wall of adult rat hearts.
Figures


References
-
- Abriel H, Wehrens XH, Benhorin J, Kerem B, Kass RS. Molecular pharmacology of the sodium channel mutation D1790G linked to the long-QT syndrome. Circulation. 2000;102:921–925. - PubMed
-
- Antzelevitch C, Yan GX, Shimizu W. Transmural dispersion of repolarization and arrhythmogenicity: the Brugada syndrome versus the long QT syndrome. Journal of Electrocardiology. 1999;32(suppl.):158–165. - PubMed
-
- Bennett PB, Yazawa K, Makita N, George ALJ. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. - PubMed
-
- Cook SJ, Chamunorwa JP, Lancaster MK, O'Neill SC. Regional differences in the regulation of intracellular sodium and in action potential configuration in rabbit left ventricle. Pflügers Archiv. 1997;433:515–522. - PubMed
-
- Dumaine R, Wang Q, Keating MT, Hartmann HA, Schwartz PJ, Brown AM, Kirsch GE. Multiple mechanisms of Na+ channel-linked long-QT syndrome. Circulation Research. 1996;78:916–924. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

