Improved hybridisation potential of oligonucleotides comprising O-methylated anhydrohexitol nucleoside congeners
- PMID: 11600707
- PMCID: PMC60215
- DOI: 10.1093/nar/29.20.4187
Improved hybridisation potential of oligonucleotides comprising O-methylated anhydrohexitol nucleoside congeners
Abstract
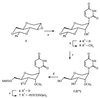
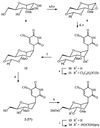
The hybridising potential of anhydrohexitol nucleoside analogues (HNAs) is well documented, but tedious synthesis of the monomers hampers their development. In a search for better analogues, the synthesis of two new methylated anhydrohexitol congeners 1 and 2 was accomplished and the physico-chemical properties of their respective oligomers were evaluated. Generally, oligonucleotides (ONs) containing the 3'-O-methyl derivative 1 showed a small increase in thermal stability towards complementary sequences as compared to HNA. Compared to the altritol modification, 3'-O-methylation seems to cause a small decrease in thermal stability of duplexes, especially when targeting RNA. These results suggest the possibility of derivatisation of the 3'-hydroxyl group of altritol-containing congeners without significantly affecting the thermal stability of the duplexes. The methyl glycosidic analogues 2 likewise increased the affinity for RNA in comparison with well-known HNA, while at the same time being economically more favorable monomers. However, homopolymers of 2 displayed self-pairing, but not so homopolymers of 1. Upon incorporation of the hexitols within RNA sequences in an effort to induce a beneficial pre-organised structure, the positive effect of the 3'-O-methyl derivative 1 proved larger than that of 2.
Figures


References
-
- Seidman S., Eckstein,F., Grifman,M. and Soreq,H. (1999) Antisense technologies have a future fighting neurodegenerative diseases. Antisense Nucleic Acid Drug Dev., 9, 333–340. - PubMed
-
- Akhtar S., Hughes,M.D., Khan,A., Bibby,M., Hussain,M., Nawaz,Q., Double,J. and Sayyed,P. (2000) The delivery of antisense therapeutics. Adv. Drug Delivery Rev., 44, 3–21. - PubMed
-
- De Mesmaeker A., Haner,R., Martin,P. and Moser,H.E. (1995) Antisense oligonucleotides. Acc. Chem. Res., 28, 366–374.
-
- Herdewijn P. (2000) Heterocyclic modifications of oligonucleotides and antisense technology. Antisense Nucleic Acid Drug Dev., 10, 297–310. - PubMed
-
- Dias N., Dheur,S., Nielsen,P.E., Gryaznov,S., Van Aerschot,A., Herdewijn,P., Helene,C. and Saison-Behmoaras,T.E. (1999) Antisense PNA tridecamers targeted to the coding region of Ha-ras mRNA arrest polypeptide chain elongation. J. Mol. Biol., 294, 403–416. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

