Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120
- PMID: 11602021
- PMCID: PMC57811
- DOI: 10.1186/1471-2334-1-17
Cellulose acetate phthalate, a common pharmaceutical excipient, inactivates HIV-1 and blocks the coreceptor binding site on the virus envelope glycoprotein gp120
Abstract
Background: Cellulose acetate phthalate (CAP), a pharmaceutical excipient used for enteric film coating of capsules and tablets, was shown to inhibit infection by the human immunodeficiency virus type 1 (HIV-1) and several herpesviruses. CAP formulations inactivated HIV-1, herpesvirus types 1 (HSV-1) and 2 (HSV-2) and the major nonviral sexually transmitted disease (STD) pathogens and were effective in animal models for vaginal infection by HSV-2 and simian immunodeficiency virus.
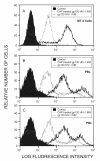
Methods: Enzyme-linked immunoassays and flow cytometry were used to demonstrate CAP binding to HIV-1 and to define the binding site on the virus envelope.
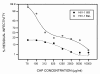
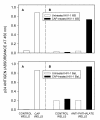
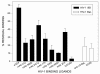
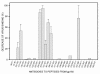
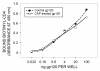
Results: 1) CAP binds to HIV-1 virus particles and to the envelope glycoprotein gp120; 2) this leads to blockade of the gp120 V3 loop and other gp120 sites resulting in diminished reactivity with HIV-1 coreceptors CXCR4 and CCR5; 3) CAP binding to HIV-1 virions impairs their infectivity; 4) these findings apply to both HIV-1 IIIB, an X4 virus, and HIV-1 BaL, an R5 virus.
Conclusions: These results provide support for consideration of CAP as a topical microbicide of choice for prevention of STDs, including HIV-1 infection.
Figures
Similar articles
-
Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides.BMC Infect Dis. 2002 Nov 21;2:27. doi: 10.1186/1471-2334-2-27. Epub 2002 Nov 21. BMC Infect Dis. 2002. PMID: 12445331 Free PMC article.
-
Anti-HIV-1 activity of cellulose acetate phthalate: synergy with soluble CD4 and induction of "dead-end" gp41 six-helix bundles.BMC Infect Dis. 2002 Apr 30;2:6. doi: 10.1186/1471-2334-2-6. BMC Infect Dis. 2002. PMID: 11983022 Free PMC article.
-
Water dispersible microbicidal cellulose acetate phthalate film.BMC Infect Dis. 2003 Nov 14;3:27. doi: 10.1186/1471-2334-3-27. BMC Infect Dis. 2003. PMID: 14617380 Free PMC article.
-
Microbicide for prevention of sexually transmitted diseases using a pharmaceutical excipient.AIDS Patient Care STDS. 2000 Apr;14(4):215-9. doi: 10.1089/108729100317830. AIDS Patient Care STDS. 2000. PMID: 10806641 Review.
-
HIV coreceptors: role of structure, posttranslational modifications, and internalization in viral-cell fusion and as targets for entry inhibitors.Biochim Biophys Acta. 2003 Jul 11;1614(1):51-61. doi: 10.1016/s0005-2736(03)00162-7. Biochim Biophys Acta. 2003. PMID: 12873765 Review.
Cited by
-
A Novel Vision of Reinforcing Nanofibrous Masks with Metal Nanoparticles: Antiviral Mechanisms Investigation.Adv Fiber Mater. 2023 Apr 11:1-45. doi: 10.1007/s42765-023-00275-7. Online ahead of print. Adv Fiber Mater. 2023. PMID: 37361103 Free PMC article. Review.
-
Combinatorial approaches to the prevention and treatment of HIV-1 infection.Antimicrob Agents Chemother. 2011 May;55(5):1831-42. doi: 10.1128/AAC.00976-10. Epub 2011 Feb 22. Antimicrob Agents Chemother. 2011. PMID: 21343462 Free PMC article. Review.
-
HIV-1 gp41-targeting fusion inhibitory peptides enhance the gp120-targeting protein-mediated inactivation of HIV-1 virions.Emerg Microbes Infect. 2017 Jun 21;6(6):e59. doi: 10.1038/emi.2017.46. Emerg Microbes Infect. 2017. PMID: 28634358 Free PMC article.
-
Anti-HIV-1 activity of anionic polymers: a comparative study of candidate microbicides.BMC Infect Dis. 2002 Nov 21;2:27. doi: 10.1186/1471-2334-2-27. Epub 2002 Nov 21. BMC Infect Dis. 2002. PMID: 12445331 Free PMC article.
-
Anti-HIV-1 activity of cellulose acetate phthalate: synergy with soluble CD4 and induction of "dead-end" gp41 six-helix bundles.BMC Infect Dis. 2002 Apr 30;2:6. doi: 10.1186/1471-2334-2-6. BMC Infect Dis. 2002. PMID: 11983022 Free PMC article.
References
-
- Lee JC. Cellulose acetate phthalate. In Handbook of Pharmaceutical Excipients Edited by Wade A, Weller PJ Washington, DC: American Pharmaceutical Association Pub; 1994. pp. 91–93.
-
- Gyotoku T, Aurelian L, Neurath AR. Cellulose acetate phthalate (CAP): an 'inactive' pharmaceutical excipient with antiviral activity in the mouse model of genital herpesvirus infection. A ntivir Chem Chemother. 1999;10:327–332. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

