Retinoic acid prevents experimental Cushing syndrome
- PMID: 11602619
- PMCID: PMC209498
- DOI: 10.1172/JCI11098
Retinoic acid prevents experimental Cushing syndrome
Abstract
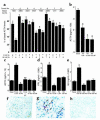
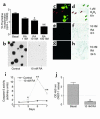
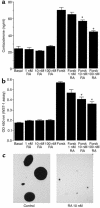
Cushing syndrome is caused by an excess of adrenocorticotropic hormone (ACTH) production by neuroendocrine tumors, which subsequently results in chronic glucocorticoid excess. We found that retinoic acid inhibits the transcriptional activity of AP-1 and the orphan receptors Nur77 and Nurr1 in ACTH-secreting tumor cells. Retinoic acid treatment resulted in reduced pro-opiomelanocortin transcription and ACTH production. ACTH inhibition was also observed in human pituitary ACTH-secreting tumor cells and a small-cell lung cancer cell line, but not in normal cells. This correlated with the expression of the orphan receptor COUP-TFI, which was found in normal corticotrophs but not in pituitary Cushing tumors. COUP-TFI expression in ACTH-secreting tumor cells blocked retinoic acid action. Retinoic acid also inhibited cell proliferation and, after prolonged treatment, increased caspase-3 activity and induced cell death in ACTH-secreting cells. In adrenal cortex cells, retinoic acid inhibited corticosterone production and cell proliferation. The antiproliferative action and the inhibition of ACTH and corticosterone produced by retinoic acid were confirmed in vivo in experimental ACTH-secreting tumors in nude mice. Thus, we conclude that the effects of retinoic acid combine in vivo to reverse the endocrine alterations and symptoms observed in experimental Cushing syndrome.
Figures
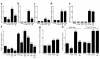


References
-
- Orth DN. Cushing’s syndrome. N Engl J Med. 1995;332:791–803. - PubMed
-
- Dahia PLM, Grossman A. The molecular pathogenesis of corticotroph tumors. Endocr Rev. 1999;20:136–155. - PubMed
-
- Wajchenberg BL, et al. Ectopic adrenocorticotropic hormone syndrome. Endocr Rev. 1994;15:752–787. - PubMed
-
- Engler D, Redei E, Kola I. The corticotropin-release inhibitory factor hypothesis: a review of the evidence for the existence of inhibitory as well as stimulatory hypophysiotropic regulation of adrenocorticotropin secretion and biosynthesis. Endocr Rev. 1999;20:460–500. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

