Antigenicity and immunogenicity of allogeneic retinal transplants
- PMID: 11602625
- PMCID: PMC209524
- DOI: 10.1172/JCI12204
Antigenicity and immunogenicity of allogeneic retinal transplants
Abstract
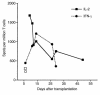
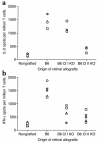
The transplantation of neuronal cells and tissues represents a promising approach for the treatment of incurable neurodegenerative diseases. Indeed, it has been reported recently that retinal transplantation can rescue photoreceptor cells and delay age-related changes in various retinal layers in rodents. However, retinal grafts deteriorate progressively after placement in recipients' eyes. Here we investigated whether a host's immune response elicited toward the graft contributes to its deterioration. Using an ELISA spot assay, we measured T cell responses to retinal tissues placed in the vitreous cavity of syngeneic and allogeneic mice. We found that allogeneic retinas induced potent alloimmune responses mediated by T cells secreting type 1 cytokines (IFN-gamma and IL-2). No response was found in mice engrafted with syngeneic retinas. In addition, all syngeneic retinal grafts displayed no signs of tissue damage (at 55 days), while the majority of allogeneic retinas deteriorated as early as 12 days after placement. Next, we showed that anti-donor responses occurred within two phenotypically and functionally distinct T cell subsets: CD4+ T cells secreting IL-2 and CD8+ T cells producing IFN-gamma. Importantly, CD4+ T cells were necessary and sufficient to cause graft deterioration, while CD8+ T cells did not contribute to this process.
Figures






References
-
- Algvere PV, Berglin L, Gouras P, Sheng Y. Transplantation of fetal retinal pigment epithelium in age-related macular degeneration with subfoveal neovascularization. Graefes Arch Clin Exp Ophthalmol. 1994;232:707–716. - PubMed
-
- del Cerro M, et al. Retinal transplants for cell replacement in phototoxic retinal degeneration. Prog Clin Biol Res. 1989;314:673–686. - PubMed
-
- del Cerro M, et al. Retinal transplants into adult eyes affected by phototoxic retinopathy. Prog Brain Res. 1988;78:125–130. - PubMed
-
- Gouras P, Flood MT, Kjedbye H, Bilek MK, Eggers H. Transplantation of cultured human retinal epithelium to Bruch’s membrane of the owl monkey’s eye. Curr Eye Res. 1985;4:253–265. - PubMed
-
- Gouras P, Lopez R. Transplantation of retinal epithelial cells. Invest Ophthalmol Vis Sci. 1989;30:1681–1683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

