Identification of aleutian mink disease parvovirus capsid sequences mediating antibody-dependent enhancement of infection, virus neutralization, and immune complex formation
- PMID: 11602751
- PMCID: PMC114691
- DOI: 10.1128/JVI.75.22.11116-11127.2001
Identification of aleutian mink disease parvovirus capsid sequences mediating antibody-dependent enhancement of infection, virus neutralization, and immune complex formation
Abstract
Aleutian mink disease parvovirus (ADV) causes a persistent infection associated with circulating immune complexes, immune complex disease, hypergammaglobulinemia, and high levels of antiviral antibody. Although antibody can neutralize ADV infectivity in Crandell feline kidney cells in vitro, virus is not cleared in vivo, and capsid-based vaccines have proven uniformly ineffective. Antiviral antibody also enables ADV to infect macrophages, the target cells for persistent infection, by Fc-receptor-mediated antibody-dependent enhancement (ADE). The antibodies involved in these unique aspects of ADV pathogenesis may have specific targets on the ADV capsid. Prominent differences exist between the structure of ADV and other, more-typical parvoviruses, which can be accounted for by short peptide sequences in the flexible loop regions of the capsid proteins. In order to determine whether these short sequences are targets for antibodies involved in ADV pathogenesis, we studied heterologous antibodies against several peptides present in the major capsid protein, VP2. Of these antibodies, a polyclonal rabbit antibody to peptide VP2:428-446 was the most interesting. The anti-VP2:428-446 antibody aggregated virus particles into immune complexes, mediated ADE, and neutralized virus infectivity in vitro. Thus, antibody against this short peptide can be implicated in key facets of ADV pathogenesis. Structural modeling suggested that surface-exposed residues of VP2:428-446 are readily accessible for antibody binding. The observation that antibodies against a single target peptide in the ADV capsid can mediate both neutralization and ADE may explain the failure of capsid-based vaccines.
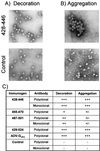
Figures

Similar articles
-
Expression of Aleutian mink disease parvovirus capsid proteins in defined segments: localization of immunoreactive sites and neutralizing epitopes to specific regions.J Virol. 1997 Jan;71(1):705-14. doi: 10.1128/JVI.71.1.705-714.1997. J Virol. 1997. PMID: 8985402 Free PMC article.
-
The relationship between capsid protein (VP2) sequence and pathogenicity of Aleutian mink disease parvovirus (ADV): a possible role for raccoons in the transmission of ADV infections.J Virol. 1996 Feb;70(2):852-61. doi: 10.1128/JVI.70.2.852-861.1996. J Virol. 1996. PMID: 8551624 Free PMC article.
-
Expression of Aleutian mink disease parvovirus capsid proteins by a recombinant vaccinia virus: self-assembly of capsid proteins into particles.J Virol. 1992 May;66(5):3077-85. doi: 10.1128/JVI.66.5.3077-3085.1992. J Virol. 1992. PMID: 1313919 Free PMC article.
-
[Eenie, Meenie, Miney, Moe, who is responsible for the antibody-dependent enhancement of Aleutian mink disease parvovirus infection?].Bing Du Xue Bao. 2014 Jul;30(4):450-5. Bing Du Xue Bao. 2014. PMID: 25272602 Review. Chinese.
-
Pathogenesis of disease caused by Aleutian mink disease parvovirus.APMIS Suppl. 1990;14:1-32. APMIS Suppl. 1990. PMID: 2159766 Review.
Cited by
-
The abundant R2 mRNA generated by aleutian mink disease parvovirus is tricistronic, encoding NS2, VP1, and VP2.J Virol. 2007 Jul;81(13):6993-7000. doi: 10.1128/JVI.00244-07. Epub 2007 Apr 11. J Virol. 2007. PMID: 17428872 Free PMC article.
-
AMDV Vaccine: Challenges and Perspectives.Viruses. 2021 Sep 14;13(9):1833. doi: 10.3390/v13091833. Viruses. 2021. PMID: 34578415 Free PMC article. Review.
-
Adeno-associated virus serotype 1 (AAV1)- and AAV5-antibody complex structures reveal evolutionary commonalities in parvovirus antigenic reactivity.J Virol. 2015 Feb;89(3):1794-808. doi: 10.1128/JVI.02710-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410874 Free PMC article.
-
B-Cell Responses to Human Bocaviruses 1-4: New Insights from a Childhood Follow-Up Study.PLoS One. 2015 Sep 29;10(9):e0139096. doi: 10.1371/journal.pone.0139096. eCollection 2015. PLoS One. 2015. PMID: 26418064 Free PMC article.
-
Structure-guided evolution of antigenically distinct adeno-associated virus variants for immune evasion.Proc Natl Acad Sci U S A. 2017 Jun 13;114(24):E4812-E4821. doi: 10.1073/pnas.1704766114. Epub 2017 May 30. Proc Natl Acad Sci U S A. 2017. PMID: 28559317 Free PMC article.
References
-
- Aasted B, Alexandersen S, Christensen J. Vaccination with Aleutian mink disease parvovirus (AMDV) capsid proteins enhances disease, while vaccination with the major non-structural AMDV protein causes partial protection from disease. Vaccine. 1998;16:1158–1165. - PubMed
-
- Aasted B, Tierney G S, Bloom M E. Analysis of the quantity of antiviral antibodies from mink infected with different Aleutian disease virus strains. Scand J Immunol. 1984;19:395–402. - PubMed
-
- Agbandje-McKenna M, Llamas-Saiz A L, Wang F, Tattersall P, Rossmann M G. Functional implications of the structure of the murine parvovirus minute virus of mice. Structure. 1998;6:1369–1381. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

