Hsp104 interacts with Hsp90 cochaperones in respiring yeast
- PMID: 11604493
- PMCID: PMC99928
- DOI: 10.1128/MCB.21.22.7569-7575.2001
Hsp104 interacts with Hsp90 cochaperones in respiring yeast
Abstract
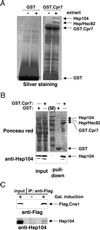
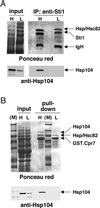
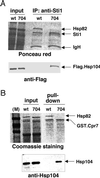
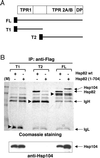
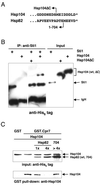
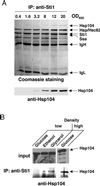
The highly abundant molecular chaperone Hsp90 functions with assistance from auxiliary factors, collectively referred to as Hsp90 cochaperones, and the Hsp70 system. Hsp104, a molecular chaperone required for stress tolerance and for maintenance of [psi(+)] prions in the budding yeast Saccharomyces cerevisiae, appears to collaborate only with the Hsp70 system. We now report that several cochaperones previously thought to be dedicated to Hsp90 are shared with Hsp104. We show that the Hsp90 cochaperones Sti1, Cpr7, and Cns1, which utilize tetratricopeptide repeat (TPR) domains to interact with a common surface on Hsp90, form complexes with Hsp104 in vivo and that Sti1 and Cpr7 interact with Hsp104 directly in vitro. The interaction is Hsp90 independent, as further emphasized by the fact that two distinct TPR domains of Sti1 are required for binding Hsp90 and Hsp104. In a striking parallel to the sequence requirements of Hsp90 for binding TPR proteins, binding of Sti1 to Hsp104 requires a related acidic sequence at the C-terminal tail of Hsp104. While Hsp90 efficiently sequesters the cochaperones during fermentative growth, respiratory conditions induce the interaction of a fraction of Hsp90 cochaperones with Hsp104. This suggests that cochaperone sharing may favor adaptation to altered metabolic conditions.
Figures
References
-
- Abbas-Terki T, Donzé O, Picard D. The molecular chaperone Cdc37 is required for Ste11 function and pheromone-induced cell cycle arrest. FEBS Lett. 2000;467:111–116. - PubMed
-
- Blum H E, Beier H, Gross H J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99.
-
- Bose S, Weikl T, Bügl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–1717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
