Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src
- PMID: 11604500
- PMCID: PMC99935
- DOI: 10.1128/MCB.21.22.7641-7652.2001
Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src
Abstract
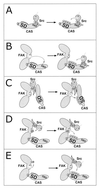
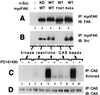
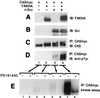
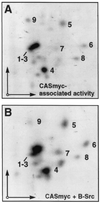
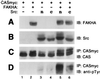
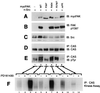
Tyrosine phosphorylation of CAS (Crk-associated substrate, p130(Cas)) has been implicated as a key signaling step in integrin control of normal cellular behaviors, including motility, proliferation, and survival. Aberrant CAS tyrosine phosphorylation may contribute to cell transformation by certain oncoproteins, including v-Crk and v-Src, and to tumor growth and metastasis. The CAS substrate domain (SD) contains 15 Tyr-X-X-Pro motifs, which are thought to represent the major tyrosine phosphorylation sites and to function by recruiting downstream signaling effectors, including c-Crk and Nck. CAS makes multiple interactions, direct and indirect, with the tyrosine kinases Src and focal adhesion kinase (FAK), and as a result of this complexity, several plausible models have been proposed for the mechanism of CAS-SD phosphorylation. The objective of this study was to provide experimental tests of these models in order to determine the most likely mechanism(s) of CAS-SD tyrosine phosphorylation by FAK and Src. In vitro kinase assays indicated that FAK has a very poor capacity to phosphorylate CAS-SD, relative to Src. However, FAK expression along with Src was found to be important for achieving high levels of CAS tyrosine phosphorylation in COS-7 cells, as well as recovery of CAS-associated Src activity toward the SD. Structure-functional studies for both FAK and CAS further indicated that FAK plays a major role in regulating CAS-SD phosphorylation by acting as a docking or scaffolding protein to recruit Src to phosphorylate CAS, while a secondary FAK-independent mechanism involves Src directly bound to the CAS Src-binding domain (SBD). Our results do not support models in which FAK either phosphorylates CAS-SD directly or phosphorylates CAS-SBD to promote Src binding to this site.
Figures


References
-
- Alexandropoulos K, Baltimore D. Coordinate activation of c-Src by SH3- and SH2-binding sites on a novel p130Cas-related protein, Sin. Genes Dev. 1996;10:1341–1355. - PubMed
-
- Astier A, Avraham H, Manie S N, Groopman J, Canty T, Avraham S, Freedman A S. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after β1 integrin stimulation in B cells and binds to p130cas. J Biol Chem. 1997;272:228–232. - PubMed
-
- Astier A, Manie S N, Avraham H, Hirai H, Law S F, Zhang Y, Golemis E A, Fu Y, Druker B J, Haghayeghi N, Freedman A S, Avraham S. The related adhesion focal tyrosine kinase differentially phosphorylates p130Cas and the Cas-like protein, p105HEF1. J Biol Chem. 1997;272:19719–19724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
