Peripheral mitochondrial inner membrane protein, Mss2p, required for export of the mitochondrially coded Cox2p C tail in Saccharomyces cerevisiae
- PMID: 11604502
- PMCID: PMC99937
- DOI: 10.1128/MCB.21.22.7663-7672.2001
Peripheral mitochondrial inner membrane protein, Mss2p, required for export of the mitochondrially coded Cox2p C tail in Saccharomyces cerevisiae
Abstract
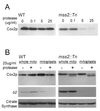
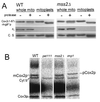
Cytochrome oxidase subunit 2 (Cox2p) is synthesized on the matrix side of the mitochondrial inner membrane, and its N- and C-terminal domains are exported across the inner membrane by distinct mechanisms. The Saccharomyces cerevisiae nuclear gene MSS2 was previously shown to be necessary for Cox2p accumulation. We have used pulse-labeling studies and the expression of the ARG8(m) reporter at the COX2 locus in an mss2 mutant to demonstrate that Mss2p is not required for Cox2p synthesis but rather for its accumulation. Mutational inactivation of the proteolytic function of the matrix-localized Yta10p (Afg3p) AAA-protease partially stabilizes Cox2p in an mss2 mutant but does not restore assembly of cytochrome oxidase. In the absence of Mss2p, the Cox2p N terminus is exported, but Cox2p C-terminal export and assembly of Cox2p into cytochrome oxidase is blocked. Epitope-tagged Mss2p is tightly, but peripherally, associated with the inner membrane and protected by it from externally added proteases. Taken together, these data indicate that Mss2p plays a role in recognizing the Cox2p C tail in the matrix and promoting its export.
Figures






References
-
- Altamura N, Capitanio N, Bonnefoy N, Papa S, Dujardin G. The Saccharomyces cerevisiae OXA1 gene is required for the correct assembly of cytochrome c oxidase and oligomycin-sensitive ATP synthase. FEBS Lett. 1996;382:111–115. - PubMed
-
- Arlt H, Tauer R, Feldmann H, Neupert W, Langer T. The YTA10-12 complex, an AAA protease with chaperone-like activity in the inner membrane of mitochondria. Cell. 1996;85:875–885. - PubMed
-
- Bauer M, Behrens M, Esser K, Michaelis G, Pratje E. PET1402, a nuclear gene required for proteolytic processing of cytochrome oxidase subunit 2 in yeast. Mol Gen Genet. 1994;245:272–278. - PubMed
-
- Blatch G L, Lassle M. The tetratricopeptide repeat: a structural motif mediating protein-protein interactions. Bioessays. 1999;21:932–939. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
