Dual function for U2AF(35) in AG-dependent pre-mRNA splicing
- PMID: 11604503
- PMCID: PMC99938
- DOI: 10.1128/MCB.21.22.7673-7681.2001
Dual function for U2AF(35) in AG-dependent pre-mRNA splicing
Abstract
The splicing factor U2AF is required for the recruitment of U2 small nuclear RNP to pre-mRNAs in higher eukaryotes. The 65-kDa subunit of U2AF (U2AF(65)) binds to the polypyrimidine (Py) tract preceding the 3' splice site, while the 35-kDa subunit (U2AF(35)) contacts the conserved AG dinucleotide at the 3' end of the intron. It has been shown that the interaction between U2AF(35) and the 3' splice site AG can stabilize U2AF(65) binding to weak Py tracts characteristic of so-called AG-dependent pre-mRNAs. U2AF(35) has also been implicated in arginine-serine (RS) domain-mediated bridging interactions with splicing factors of the SR protein family bound to exonic splicing enhancers (ESE), and these interactions can also stabilize U2AF(65) binding. Complementation of the splicing activity of nuclear extracts depleted of U2AF by chromatography in oligo(dT)-cellulose requires, for some pre-mRNAs, only the presence of U2AF(65). In contrast, splicing of a mouse immunoglobulin M (IgM) M1-M2 pre-mRNA requires both U2AF subunits. In this report we have investigated the sequence elements (e.g., Py tract strength, 3' splice site AG, ESE) responsible for the U2AF(35) dependence of IgM. The results indicate that (i) the IgM substrate is an AG-dependent pre-mRNA, (ii) U2AF(35) dependence correlates with AG dependence, and (iii) the identity of the first nucleotide of exon 2 is important for U2AF(35) function. In contrast, RS domain-mediated interactions with SR proteins bound to the ESE appear to be dispensable, because the purine-rich ESE present in exon M2 is not essential for U2AF(35) activity and because a truncation mutant of U2AF(35) consisting only of the pseudo-RNA recognition motif domain and lacking the RS domain is active in our complementation assays. While some of the effects of U2AF(35) can be explained in terms of enhanced U2AF(65) binding, other activities of U2AF(35) do not correlate with increased cross-linking of U2AF(65) to the Py tract. Collectively, the results argue that interaction of U2AF(35) with a consensus 3' splice site triggers events in spliceosome assembly in addition to stabilizing U2AF(65) binding, thus revealing a dual function for U2AF(35) in pre-mRNA splicing.
Figures


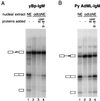
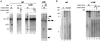

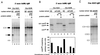

Similar articles
-
Distinct factor requirements for exonic splicing enhancer function and binding of U2AF to the polypyrimidine tract.J Biol Chem. 1999 Dec 3;274(49):35074-9. doi: 10.1074/jbc.274.49.35074. J Biol Chem. 1999. PMID: 10574987
-
Evidence for substrate-specific requirement of the splicing factor U2AF(35) and for its function after polypyrimidine tract recognition by U2AF(65).Mol Cell Biol. 1999 Dec;19(12):8263-71. doi: 10.1128/MCB.19.12.8263. Mol Cell Biol. 1999. PMID: 10567551 Free PMC article.
-
Substrate-dependent differences in U2AF requirement for splicing in adenovirus-infected cell extracts.J Biol Chem. 2005 Jul 8;280(27):25478-84. doi: 10.1074/jbc.M413737200. Epub 2005 May 16. J Biol Chem. 2005. PMID: 15899895
-
Mechanisms of fidelity in pre-mRNA splicing.Curr Opin Cell Biol. 2000 Jun;12(3):340-5. doi: 10.1016/s0955-0674(00)00097-1. Curr Opin Cell Biol. 2000. PMID: 10801464 Review.
-
U2AF homology motifs: protein recognition in the RRM world.Genes Dev. 2004 Jul 1;18(13):1513-26. doi: 10.1101/gad.1206204. Genes Dev. 2004. PMID: 15231733 Free PMC article. Review.
Cited by
-
Analysis of mutant phenotypes and splicing defects demonstrates functional collaboration between the large and small subunits of the essential splicing factor U2AF in vivo.Mol Biol Cell. 2005 Feb;16(2):584-96. doi: 10.1091/mbc.e04-09-0768. Epub 2004 Nov 17. Mol Biol Cell. 2005. PMID: 15548596 Free PMC article.
-
The splicing factor U2AF small subunit is functionally conserved between fission yeast and humans.Mol Cell Biol. 2004 May;24(10):4229-40. doi: 10.1128/MCB.24.10.4229-4240.2004. Mol Cell Biol. 2004. PMID: 15121844 Free PMC article.
-
mTOR-regulated U2af1 tandem exon splicing specifies transcriptome features for translational control.Nucleic Acids Res. 2019 Nov 4;47(19):10373-10387. doi: 10.1093/nar/gkz761. Nucleic Acids Res. 2019. PMID: 31504847 Free PMC article.
-
In vivo requirement of the small subunit of U2AF for recognition of a weak 3' splice site.Mol Cell Biol. 2006 Nov;26(21):8183-90. doi: 10.1128/MCB.00350-06. Epub 2006 Aug 28. Mol Cell Biol. 2006. PMID: 16940179 Free PMC article.
-
Allele-specific recognition of the 3' splice site of INS intron 1.Hum Genet. 2010 Oct;128(4):383-400. doi: 10.1007/s00439-010-0860-1. Epub 2010 Jul 14. Hum Genet. 2010. PMID: 20628762 Free PMC article.
References
-
- Amrein H, Hedley M, Maniatis T. The role of specific protein-RNA and protein-protein interactions in positive and negative control of pre-mRNA splicing by Transformer-2. Cell. 1994;76:735–746. - PubMed
-
- Berglund J, Chua K, Abovich N, Reed R, Rosbash M. The splicing factor BBP interacts specifically with the pre-mRNA branchpoint sequence UACUAAC. Cell. 1997;89:781–787. - PubMed
-
- Blencowe B. Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases. Trends Biochem Sci. 2000;25:106–110. - PubMed
-
- Bouck J, Fu X, Skalka A, Katz R. Role of the constitutive splicing factors U2AF65 and SAP49 in suboptimal RNA splicing of novel retroviral mutants. J Biol Chem. 1998;273:15169–15176. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
