Pravastatin suppresses the interleukin-8 production induced by thrombin in human aortic endothelial cells cultured with high glucose by inhibiting the p44/42 mitogen activated protein kinase
- PMID: 11606315
- PMCID: PMC1572999
- DOI: 10.1038/sj.bjp.0704305
Pravastatin suppresses the interleukin-8 production induced by thrombin in human aortic endothelial cells cultured with high glucose by inhibiting the p44/42 mitogen activated protein kinase
Abstract
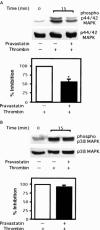
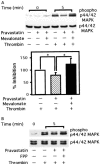
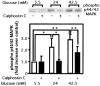
1. 3-Hydroxy-3-methylglutaryl co-enzyme A reductase inhibitors (statins) prevent the progression of atherosclerosis by lowering cholesterol. However, the effect of statins on the synthesis of pro-inflammatory cytokines from endothelial cells has not yet been fully investigated. Here, we examined the effect of pravastatin, one of the statins, on IL-8 synthesis induced by thrombin in human aortic endothelial cells (AoEC) cultured with high glucose concentrations. 2. Pravastatin significantly decreased the IL-8 synthesis induced by thrombin. 3. Pravastatin inhibited the p44/42 MAP kinase activity induced by thrombin, but did not inhibit the p38 MAP kinase activity. 4. Translocation of ras protein from the cytosol to plasma membrane was inhibited by pravastatin. 5. Pravastatin inhibit the activator protein-1 activity, but did not inhibit the activation of IkappaB-alpha. 6. Dominant negative ras inhibited the p44/42 MAP kinase activity induced by PMA. 7. Our results suggest that pravastatin inhibits IL-8 synthesis by blocking the ras-MAP (p44/42) kinase pathway rather than nuclear factor-kappaB. Pravastatin may prevent atherosclerosis not only by lowering cholesterol levels, but also by suppressing IL-8 synthesis in AoEC through the inhibition of p44/42 MAP kinase, and this may be more beneficial in diabetic patients than in non-diabetics.
Figures










References
-
- ANRATHER D., MILLAN M.T., PALMETSHOFER A., ROBSON S.C., GECZY C., RITCHIE A.J., BACH F.H., EWENSTEIN B.M. Thrombin activates nuclear factor-kappaB and potentiates endothelial cell activation by TNF. J. Immunol. 1997;159:5620–5628. - PubMed
-
- BACON K.B., WESTWICK J., CAMP R.D. Potent and specific inhibition of IL-8-, IL-1 alpha- and IL-1 beta-induced in vitro human lymphocyte migration by calcium channel antagonists. Biochem. Biophys. Res. Commun. 1989;165:349–354. - PubMed
-
- CERIELLO A., GIACOMELLO R., COLATUTTO A., TABOGA C., GONANO F. Increased prothrombin fragment 1+2 in type I diabetic patients. Haemostasis. 1992;22:50–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

