Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes
- PMID: 11606430
- PMCID: PMC4446976
- DOI: 10.1210/endo.142.11.8510
Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes
Abstract
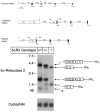
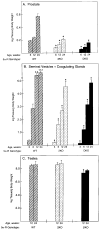
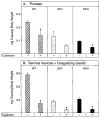
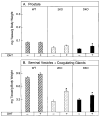
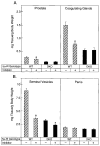
Mice lacking steroid 5 alpha-reductase 1 and 2 were produced by gene targeting and breeding. Male mice without 5 alpha-reductase 2 or without both enzymes had fully formed internal and external genitalia and were fertile, but had smaller prostates and seminal vesicles than controls. T accumulated to high levels in the reproductive tissues of the mutant mice. DHT administration increased seminal vesicle and coagulating gland weights in mice deficient in 5 alpha-reductase 2 and increased the weights of the prostate, seminal vesicle, and coagulating gland in animals deficient in both enzymes. An inhibitor of both 5 alpha-reductases (GI 208335X) decreased prostate and coagulating gland weights of control mice, but had no effect in those lacking 5 alpha-reductase 1 and 2. Castration reduced the sizes of these tissues in animals of all genotypes. Androgen-dependent gene expression was decreased in the seminal vesicles of mice lacking one or more 5 alpha-reductases and was restored by administration of T or DHT. Female mice missing both enzymes exhibited parturition and fecundity defects similar to those of animals without 5 alpha-reductase 1. We conclude that T is the only androgen required for differentiation of the male urogenital tract in mice and that the synthesis of DHT serves largely as a signal amplification mechanism.
Figures


Similar articles
-
Comparison of the effects of the 5 alpha-reductase inhibitor finasteride and the antiandrogen flutamide on prostate and genital differentiation: dose-response studies.Endocrinology. 1992 Sep;131(3):1149-56. doi: 10.1210/endo.131.3.1324152. Endocrinology. 1992. PMID: 1324152
-
The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity.J Clin Endocrinol Metab. 1993 Feb;76(2):524-8. doi: 10.1210/jcem.76.2.8381804. J Clin Endocrinol Metab. 1993. PMID: 8381804
-
Expression and regulation of steroid 5 alpha-reductase in the urogenital tract of the fetal rat.Mol Endocrinol. 1995 Nov;9(11):1561-70. doi: 10.1210/mend.9.11.8584033. Mol Endocrinol. 1995. PMID: 8584033
-
Androgens and male physiology the syndrome of 5alpha-reductase-2 deficiency.Mol Cell Endocrinol. 2002 Dec 30;198(1-2):51-9. doi: 10.1016/s0303-7207(02)00368-4. Mol Cell Endocrinol. 2002. PMID: 12573814 Review.
-
Steroid 5α-reductase 2 deficiency.J Steroid Biochem Mol Biol. 2016 Oct;163:206-11. doi: 10.1016/j.jsbmb.2016.05.020. Epub 2016 May 22. J Steroid Biochem Mol Biol. 2016. PMID: 27224879 Review.
Cited by
-
Intracrine Regulation of Estrogen and Other Sex Steroid Levels in Endometrium and Non-gynecological Tissues; Pathology, Physiology, and Drug Discovery.Front Pharmacol. 2018 Sep 19;9:940. doi: 10.3389/fphar.2018.00940. eCollection 2018. Front Pharmacol. 2018. PMID: 30283331 Free PMC article. Review.
-
Semiquantitative RT-PCR method coupled to capillary electrophoresis to study 5alpha-reductase mRNA isozymes in rat ventral prostate in different androgen status.Mol Cell Biochem. 2003 Aug;250(1-2):125-30. doi: 10.1023/a:1024902419502. Mol Cell Biochem. 2003. PMID: 12962150
-
Loss of 5α-reductase type 1 accelerates the development of hepatic steatosis but protects against hepatocellular carcinoma in male mice.Endocrinology. 2013 Dec;154(12):4536-47. doi: 10.1210/en.2013-1592. Epub 2013 Sep 30. Endocrinology. 2013. PMID: 24080367 Free PMC article.
-
Possible testosterone redundancy for 5α-dihydrotestosterone in the masculinization of mouse external genitalia.Exp Anim. 2022 Nov 10;71(4):451-459. doi: 10.1538/expanim.22-0038. Epub 2022 May 24. Exp Anim. 2022. PMID: 35613877 Free PMC article.
-
The androgen clock is an epigenetic predictor of long-term male hormone exposure.Proc Natl Acad Sci U S A. 2025 Jan 21;122(3):e2420087121. doi: 10.1073/pnas.2420087121. Epub 2025 Jan 13. Proc Natl Acad Sci U S A. 2025. PMID: 39805019 Free PMC article.
References
-
- Wilson JD. Metabolism of testicular androgens. Handb Physiol. 1975;5:491–508.
-
- Andersson S, Russell DW, Wilson JD. 17β-Hydroxysteroid dehydrogenase 3 deficiency. Trends Endocrinol Metab. 1996;7:121–126. - PubMed
-
- Walsh PC, Madden JD, Harrod MJ, Goldstein JL, MacDonald PC, Wilson JD. Familial incomplete male pseudohermaphroditism, type 2. Decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med. 1974;291:944–949. - PubMed
-
- Imperato-McGinley J, Guerrero JL, Gautier T, Peterson RE. Steroid 5α-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186:1213–1215. - PubMed
-
- Imperato-McGinley J, Binienda Z, Arthur A, Mininberg DT, Vaughan ED, Jr, Quimby FW. The development of a male pseudohermaphroditic rat using an inhibitor of the enzyme 5α-reductase. Endocrinology. 1985;16:807–812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

