Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson's disease
- PMID: 11606633
- PMCID: PMC6762816
- DOI: 10.1523/JNEUROSCI.21-21-08447.2001
Microglial activation and dopaminergic cell injury: an in vitro model relevant to Parkinson's disease
Abstract
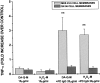
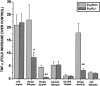
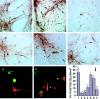
Microglial activation and oxidative stress are significant components of the pathology of Parkinson's disease (PD), but their exact contributions to disease pathogenesis are unclear. We have developed an in vitro model of nigral injury, in which lipopolysaccharide-induced microglial activation leads to injury of a dopaminergic cell line (MES 23.5 cells) and dopaminergic neurons in primary mesencephalic cell cultures. The microglia are also activated by PD IgGs in the presence of low-dose dopa-quinone- or H(2)O(2)-modified dopaminergic cell membranes but not cholinergic cell membranes. The activation requires the microglial FCgammaR receptor as demonstrated by the lack of activation with PD IgG Fab fragments or microglia from FCgammaR-/- mice. Although microglial activation results in the release of several cytokines and reactive oxygen species, only nitric oxide and H(2)O(2) appear to mediate the microglia-induced dopaminergic cell injury. These studies suggest a significant role for microglia in dopaminergic cell injury and provide a mechanism whereby immune/inflammatory reactions in PD could target oxidative injury relatively specifically to dopaminergic cells.
Figures





References
-
- Appel SH, Le WD, Tajti J, Haverkamp LJ, Engelhardt JI. Nigral damage and dopaminergic hypofunction in mesencephalon-immunized guinea pigs. Ann Neurol. 1992;32:494–501. - PubMed
-
- Beal MF. Excitotoxicity and nitric oxide in Parkinson's disease pathogenesis. Ann Neurol. 1998;44[Suppl 1]:S110–S114. - PubMed
-
- Bencsik A, Akaoka H, Giraudon P, Belin MF, Bernard A. Inhibition of tyrosine hydroxylase expression within the substantia nigra of mice infected with canine distemper virus. J Neuropathol Exp Neurol. 1997;56:673–685. - PubMed
-
- Bianca VD, Dusi S, Bianchin E, Pra ID, Rossi F. β-Amyloid activates the O2- forming NADPH oxidase in microglia, monocytes, and neurotrophis. J Biol Chem. 1999;274:15493–15499. - PubMed
-
- Brundin P, Karlsson J, Emgard M, Schierle GS, Hansson O, Petersen A, Castilho RH. Improving the survival of grafted dopaminergic neurons: a review over current approaches. Cell Transplant. 2000;9:179–195. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
