Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb
- PMID: 11606639
- PMCID: PMC6762814
- DOI: 10.1523/JNEUROSCI.21-21-08505.2001
Phenotypic differentiation during migration of dopaminergic progenitor cells to the olfactory bulb
Abstract
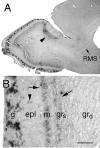
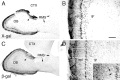
A possible source for transplantable neurons in Parkinson's disease are adult olfactory bulb (OB) dopamine (DA) progenitors that originate in the anterior subventricular zone and reach the OB through the rostral migratory stream. We used adult transgenic mice expressing a lacZ reporter directed by an 8.9 kb tyrosine hydroxylase (TH) promoter to investigate the course of DAergic differentiation. Parallel transgene and intrinsic TH mRNA expression occurred during migration of DA interneurons through the mitral and superficial granule cell layers before these cells reached their final periglomerular position. Differential transgene and calcium-calmodulin-dependent protein kinase IV expression distinguished two nonoverlapping populations of interneurons. Transgenic mice carrying a TH8.9kb/lacZ construct with a mutant AP-1 site demonstrated that this element confers OB DA-specific TH gene regulation. These results indicate that DA phenotypic determination is specific to a subset of mobile OB progenitors.
Figures





References
-
- Alvarez-Buylla A, Temple S. Stem cells in the developing and adult nervous system. J Neurobiol. 1998;36:105–110. - PubMed
-
- Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. - PubMed
-
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997a;278:474–476. - PubMed
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997b;19:27–37. - PubMed
-
- Baker H. Unilateral, neonatal olfactory deprivation alters tyrosine hydroxylase expression but not aromatic amino acid decarboxylase or GABA immunoreactivity. Neuroscience. 1990;36:761–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
