Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones
- PMID: 11606642
- PMCID: PMC6762807
- DOI: 10.1523/JNEUROSCI.21-21-08538.2001
Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones
Abstract
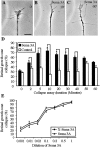
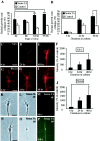
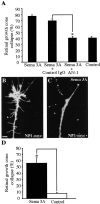
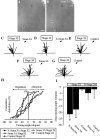
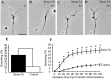
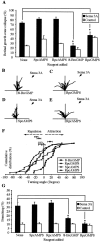
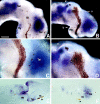
The semaphorin receptor, neuropilin-1 (NP-1), was first identified in Xenopus as the A5 antigen and is expressed abundantly in developing retinal ganglion cells (RGCs). Here we show that growth cones acquire responsiveness to semaphorin 3A (Sema 3A) with age and that the onset of responsiveness correlates with the appearance of NP-1 immunoreactivity. Growth cones from "old" (stage 35/36) retinal explants collapse rapidly (5-10 min) in response to Sema 3A and turn away from a gradient of Sema 3A, whereas "young" growth cones (stage 24) are insensitive to Sema 3A. Moreover, transfection of full-length NP-1 into young neurons confers premature Sema 3A sensitivity. When young neurons are aged in culture they develop Sema 3A sensitivity in parallel with those in vivo, suggesting that an intrinsic mechanism of NP-1 regulation mediates this age-dependent change. Sema 3A-induced collapse is transient, and after recovery approximately 30% of growth cones extend new branches within 1 hr, implicating Sema 3A as a branching factor. Pharmacological inhibitors were used to investigate whether these three Sema 3A-induced behaviors (collapse, turning, and branching) use distinct second messenger signaling pathways. All three behaviors were found to be mediated via cGMP. In situ hybridization shows that Sema 3A is expressed in the tectum and at the anterior boundary of the optic tract where axons bend caudally, suggesting that Sema 3A/NP-1 interactions play a role in guiding axons in the optic tract and in stimulating terminal branching in the tectum.
Figures
References
-
- Acebes A, Ferrus A. Cellular and molecular features of axon collaterals and dendrites. Trends Neurosci. 2000;23:557–565. - PubMed
-
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. - PubMed
-
- Brose K, Tessier-Lavigne M. Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr Opin Neurobiol. 2000;10:95–102. - PubMed
-
- Chien CB, Harris WA. Axonal guidance from retina to tectum in embryonic Xenopus. Curr Top Dev Biol. 1994;29:135–169. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
