Transforming growth factor beta (TGFbeta) mediates Schwann cell death in vitro and in vivo: examination of c-Jun activation, interactions with survival signals, and the relationship of TGFbeta-mediated death to Schwann cell differentiation
- PMID: 11606645
- PMCID: PMC6762809
- DOI: 10.1523/JNEUROSCI.21-21-08572.2001
Transforming growth factor beta (TGFbeta) mediates Schwann cell death in vitro and in vivo: examination of c-Jun activation, interactions with survival signals, and the relationship of TGFbeta-mediated death to Schwann cell differentiation
Abstract
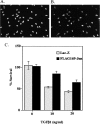
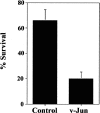
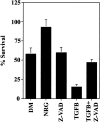
In some situations, cell death in the nervous system is controlled by an interplay between survival factors and negative survival signals that actively induce apoptosis. The present work indicates that the survival of Schwann cells is regulated by such a dual mechanism involving the negative survival signal transforming growth factor beta (TGFbeta), a family of growth factors that is present in the Schwann cells themselves. We analyze the interactions between this putative autocrine death signal and previously defined paracrine and autocrine survival signals and show that expression of a dominant negative c-Jun inhibits TGFbeta-induced apoptosis. This and other findings pinpoint activation of c-Jun as a key downstream event in TGFbeta-induced Schwann cell death. The ability of TGFbeta to kill Schwann cells, like normal Schwann cell death in vivo, is under a strong developmental regulation, and we show that the decreasing ability of TGFbeta to kill older cells is attributable to a decreasing ability of TGFbeta to phosphorylate c-Jun in more differentiated cells.
Figures

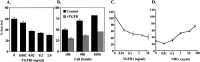

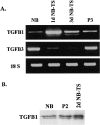

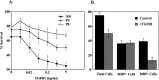

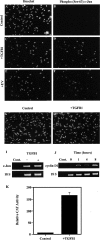

Similar articles
-
beta-Neuregulin and autocrine mediated survival of Schwann cells requires activity of Ets family transcription factors.Mol Cell Neurosci. 2002 May;20(1):154-67. doi: 10.1006/mcne.2002.1109. Mol Cell Neurosci. 2002. PMID: 12056846
-
Krox-20 inhibits Jun-NH2-terminal kinase/c-Jun to control Schwann cell proliferation and death.J Cell Biol. 2004 Feb 2;164(3):385-94. doi: 10.1083/jcb.200307132. J Cell Biol. 2004. PMID: 14757751 Free PMC article.
-
Insulin-like growth factor-I and Bcl-X(L) inhibit c-jun N-terminal kinase activation and rescue Schwann cells from apoptosis.J Neurochem. 2001 Feb;76(3):935-43. doi: 10.1046/j.1471-4159.2001.00110.x. J Neurochem. 2001. PMID: 11158266
-
Neuregulin-1, a key axonal signal that drives Schwann cell growth and differentiation.Glia. 2008 Nov 1;56(14):1491-1497. doi: 10.1002/glia.20753. Glia. 2008. PMID: 18803318 Review.
-
Schwann cells as regulators of nerve development.J Physiol Paris. 2002 Jan-Mar;96(1-2):17-24. doi: 10.1016/s0928-4257(01)00076-6. J Physiol Paris. 2002. PMID: 11755779 Review.
Cited by
-
Contribution of persistent C-Jun N-terminal kinase activity to the survival of human vestibular schwannoma cells by suppression of accumulation of mitochondrial superoxides.Neuro Oncol. 2011 Sep;13(9):961-73. doi: 10.1093/neuonc/nor068. Epub 2011 Jun 22. Neuro Oncol. 2011. PMID: 21697181 Free PMC article.
-
TGFbeta type II receptor signaling controls Schwann cell death and proliferation in developing nerves.J Neurosci. 2006 Aug 16;26(33):8417-27. doi: 10.1523/JNEUROSCI.1578-06.2006. J Neurosci. 2006. PMID: 16914667 Free PMC article.
-
The Role of c-Jun and Autocrine Signaling Loops in the Control of Repair Schwann Cells and Regeneration.Front Cell Neurosci. 2022 Feb 9;15:820216. doi: 10.3389/fncel.2021.820216. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35221918 Free PMC article. Review.
-
Laminins in peripheral nerve development and muscular dystrophy.Mol Neurobiol. 2007 Jun;35(3):288-97. doi: 10.1007/s12035-007-0026-x. Mol Neurobiol. 2007. PMID: 17917117 Review.
-
Development of myelinating glia: An overview.Glia. 2022 Dec;70(12):2237-2259. doi: 10.1002/glia.24238. Epub 2022 Jul 4. Glia. 2022. PMID: 35785432 Free PMC article. Review.
References
-
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem. 1995;270:23589–23597. - PubMed
-
- Bader AG, Hartl M, Bister K. Conditional cell transformation by doxycycline-controlled expression of the ASN17 v-Jun allele. Virology. 2000;270:98–110. - PubMed
-
- Berkner KL. Development of adenovirus vectors for the expression of heterologous genes. Biotechniques. 1998;6:616–629. - PubMed
-
- Black EJ, Catling AD, Woodgett JR, Kilbey A, Gillespie DA. Transcriptional activation by the v-Jun oncoprotein is independent of positive regulatory phosphorylation. Oncogene. 1994;9:2363–2368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
