Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety
- PMID: 11606648
- PMCID: PMC6762821
- DOI: 10.1523/JNEUROSCI.21-21-08602.2001
Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety
Abstract
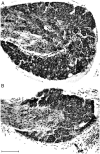
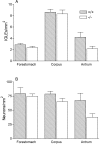
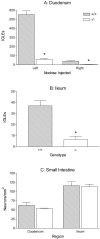
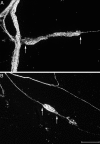
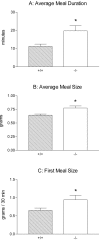
Intraganglionic laminar endings (IGLEs) and intramuscular arrays (IMAs) are the two putative mechanoreceptors that the vagus nerve supplies to gastrointestinal smooth muscle. To examine whether neurotrophin-4 (NT-4)-deficient mice, which have only 45% of the normal number of nodose ganglion neurons, exhibit selective losses of these endings and potentially provide a model for assessing their functional roles, we inventoried IGLEs and IMAs in the gut wall. Vagal afferents were labeled by nodose ganglion injections of wheat germ agglutinin-horseradish peroxidase, and a standardized sampling protocol was used to map the terminals in the stomach, duodenum, and ileum. NT-4 mutants had a substantial organ-specific reduction of IGLEs; whereas the morphologies and densities of both IGLEs and IMAs in the stomach were similar to wild-type patterns, IGLEs were largely absent in the small intestine (90 and 81% losses in duodenum and ileum, respectively). Meal pattern analyses revealed that NT-4 mutants had increased meal durations with solid food and increased meal sizes with liquid food. However, daily total food intake and body weight remained normal because of compensatory changes in other meal parameters. These findings indicate that NT-4 knock-out mice have a selective vagal afferent loss and suggest that intestinal IGLEs (1) may participate in short-term satiety, probably by conveying feedback about intestinal distension or transit to the brain, (2) are not essential for long-term control of feeding and body weight, and (3) play different roles in regulation of solid and liquid diet intake.
Figures






Similar articles
-
Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c-Kit receptor ligand.Anat Embryol (Berl). 2002 Jul;205(4):325-42. doi: 10.1007/s00429-002-0261-x. Epub 2002 Jun 15. Anat Embryol (Berl). 2002. PMID: 12136263
-
Vagal afferent innervation of smooth muscle in the stomach and duodenum of the mouse: morphology and topography.J Comp Neurol. 2000 Dec 18;428(3):558-76. doi: 10.1002/1096-9861(20001218)428:3<558::aid-cne11>3.0.co;2-m. J Comp Neurol. 2000. PMID: 11074451
-
C-Kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach.Anat Embryol (Berl). 2001 Jul;204(1):11-26. doi: 10.1007/s004290100184. Anat Embryol (Berl). 2001. PMID: 11506430
-
Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology.Brain Res Brain Res Rev. 2000 Nov;34(1-2):1-26. doi: 10.1016/s0165-0173(00)00036-9. Brain Res Brain Res Rev. 2000. PMID: 11086184 Review.
-
Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract.Neurogastroenterol Motil. 2004 Apr;16 Suppl 1:28-33. doi: 10.1111/j.1743-3150.2004.00471.x. Neurogastroenterol Motil. 2004. PMID: 15066001 Review.
Cited by
-
Nav1.8 neurons are involved in limiting acute phase responses to dietary fat.Mol Metab. 2017 Oct;6(10):1081-1091. doi: 10.1016/j.molmet.2017.07.012. Epub 2017 Aug 4. Mol Metab. 2017. PMID: 29031710 Free PMC article. Review.
-
Evaluation of study design variables and their impact on food-maintained operant responding in mice.Behav Brain Res. 2010 Mar 5;207(2):394-401. doi: 10.1016/j.bbr.2009.10.025. Epub 2009 Oct 29. Behav Brain Res. 2010. PMID: 19879302 Free PMC article.
-
You Are What You (First) Eat.Front Hum Neurosci. 2018 Aug 13;12:323. doi: 10.3389/fnhum.2018.00323. eCollection 2018. Front Hum Neurosci. 2018. PMID: 30150928 Free PMC article. Review.
-
Loss of neurotrophin-3 from smooth muscle disrupts vagal gastrointestinal afferent signaling and satiation.Am J Physiol Regul Integr Comp Physiol. 2013 Dec;305(11):R1307-22. doi: 10.1152/ajpregu.00337.2013. Epub 2013 Sep 25. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 24068045 Free PMC article.
-
Vagal Sensory Neuron Subtypes that Differentially Control Breathing.Cell. 2015 Apr 23;161(3):622-633. doi: 10.1016/j.cell.2015.03.022. Epub 2015 Apr 16. Cell. 2015. PMID: 25892222 Free PMC article.
References
-
- Aldskogius H, Elfvin LG, Forsman CA. Primary sensory afferents in the inferior mesenteric ganglion and related nerves of the guinea pig. An experimental study with anterogradely transported wheat germ agglutinin-horseradish peroxidase conjugate. J Auton Nerv Syst. 1986;15:179–190. - PubMed
-
- Azpiroz F, Malagelada JR. Perception and reflex relaxation of the stomach in response to gut distention. Gastroenterology. 1990;98:1193–1198. - PubMed
-
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. J Comp Neurol. 1992;319:261–276. - PubMed
-
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anat Embryol. 1997a;195:183–191. - PubMed
-
- Berthoud HR, Patterson LM, Willing AE, Mueller K, Neuhuber WL. Capsaicin-resistant vagal afferent fibers in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Res. 1997b;746:195–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
