Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion
- PMID: 11606656
- PMCID: PMC6762779
- DOI: 10.1523/JNEUROSCI.21-21-08680.2001
Altered respiratory motor drive after spinal cord injury: supraspinal and bilateral effects of a unilateral lesion
Abstract
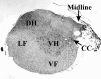
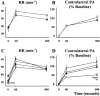
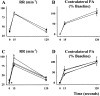
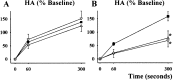
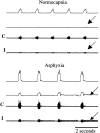
Because some bulbospinal respiratory premotor neurons have bilateral projections to the phrenic nuclei, we investigated whether changes in contralateral phrenic motoneuron function would occur after unilateral axotomy via C(2) hemisection. Phrenic neurograms were recorded under baseline conditions and during hypercapnic and hypoxic challenge in C(2) hemisected, normal, and sham-operated rats at 1 and 2 months after injury. The rats were anesthetized, vagotomized, and mechanically ventilated. No group differences were seen in contralateral neurograms at 1 month after injury. At 2 months, however, there was a statistically significant decrease in respiratory rate (RR) at normocapnia, an elevated RR during hypoxia, and an attenuated increase in phrenic neurogram amplitude during hypercapnia in the C(2)-hemisected animals. To test whether C(2) hemisection had induced a supraspinal change in respiratory motor drive, we recorded ipsilateral and contralateral hypoglossal neurograms during hypercapnia. As with the phrenic motor function data, no change in hypoglossal output was evident until 2 months had elapsed when hypoglossal amplitudes were significantly decreased bilaterally. Last, the influence of serotonin-containing neurons on the injury-induced change in phrenic motoneuron function was examined in rats treated with the serotonin neurotoxin, 5,7-dihydroxytryptamine. Pretreatment with 5,7-dihydroxytryptamine prevented the effects of C(2) hemisection on contralateral phrenic neurogram amplitude and normalized the change in RR during hypoxia. The results of this study show novel neuroplastic changes in segmental and brainstem respiratory motor output after C(2) hemisection that coincided with the spontaneous recovery of some ipsilateral phrenic function. Some of these effects may be modulated by serotonin-containing neurons.
Figures
Similar articles
-
Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output.J Neurosci. 2003 Mar 15;23(6):2494-501. doi: 10.1523/JNEUROSCI.23-06-02494.2003. J Neurosci. 2003. PMID: 12657710 Free PMC article.
-
Influence of vagal afferents on supraspinal and spinal respiratory activity following cervical spinal cord injury in rats.J Appl Physiol (1985). 2010 Aug;109(2):377-87. doi: 10.1152/japplphysiol.01429.2009. Epub 2010 May 27. J Appl Physiol (1985). 2010. PMID: 20507963 Free PMC article.
-
Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury.J Neurosci. 2005 Mar 16;25(11):2925-32. doi: 10.1523/JNEUROSCI.0148-05.2005. J Neurosci. 2005. PMID: 15772352 Free PMC article.
-
Descending bulbospinal pathways and recovery of respiratory motor function following spinal cord injury.Respir Physiol Neurobiol. 2009 Nov 30;169(2):115-22. doi: 10.1016/j.resp.2009.08.004. Epub 2009 Aug 12. Respir Physiol Neurobiol. 2009. PMID: 19682608 Review.
-
Serotonergic innervation of respiratory motor nuclei after cervical spinal injury: Impact of intermittent hypoxia.Exp Neurol. 2021 Apr;338:113609. doi: 10.1016/j.expneurol.2021.113609. Epub 2021 Jan 15. Exp Neurol. 2021. PMID: 33460645 Free PMC article. Review.
Cited by
-
Serotonergic transmission after spinal cord injury.J Neural Transm (Vienna). 2015 Feb;122(2):279-95. doi: 10.1007/s00702-014-1241-z. Epub 2014 May 28. J Neural Transm (Vienna). 2015. PMID: 24866695 Review.
-
The crossed phrenic phenomenon.Neural Regen Res. 2017 Jun;12(6):845-864. doi: 10.4103/1673-5374.208539. Neural Regen Res. 2017. PMID: 28761411 Free PMC article. Review.
-
Intermittent hypoxia induces functional recovery following cervical spinal injury.Respir Physiol Neurobiol. 2009 Nov 30;169(2):210-7. doi: 10.1016/j.resp.2009.07.023. Epub 2009 Aug 3. Respir Physiol Neurobiol. 2009. PMID: 19651247 Free PMC article. Review.
-
Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors.Respir Physiol Neurobiol. 2011 Oct 15;179(1):57-63. doi: 10.1016/j.resp.2011.06.028. Epub 2011 Jul 6. Respir Physiol Neurobiol. 2011. PMID: 21763470 Free PMC article. Review.
-
Chronic assessment of diaphragm muscle EMG activity across motor behaviors.Respir Physiol Neurobiol. 2011 Jul 31;177(2):176-82. doi: 10.1016/j.resp.2011.03.011. Epub 2011 Mar 15. Respir Physiol Neurobiol. 2011. PMID: 21414423 Free PMC article.
References
-
- Bach KB, Mitchell GS. Hypercapnia-induced long-term depression of respiratory activity requires alpha2-adrenergic receptors. J Appl Physiol. 1998;84:2099–2105. - PubMed
-
- Bach KB, Mitchell GS. Effects of phrenicotomy and exercise on hypoxia-induced changes in phrenic motor output. J Appl Physiol. 2000;89:1884–1891. - PubMed
-
- Bach KB, Kinkead R, Mitchell GS. Post-hypoxia frequency decline in rats: sensitivity to repeated hypoxia and alpha2-adrenoreceptor antagonism. Brain Res. 1999;817:25–33. - PubMed
-
- Bach KB, Johnson RA, Kinkead RK, Fuller DD, Zhan W, Mantilla C, Sieck GS, Mitchell GS. Cervical dorsal rhizotomy (CDR) enhances serotonin-dependent long-term facilitation of hypoglossal motor output in rats. FASEB J. 2000;14:A77.
-
- Baumgarten HG, Bjorklund A, Lachenmayer L, Nobin A. Evaluation of the effects of 5,7-dihydroxytryptamine on serotonin and catecholamine neurons in the CNS. Acta Physiol Scand [Suppl] 1973;391:1–19. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
