Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia
- PMID: 11606717
- PMCID: PMC60081
- DOI: 10.1073/pnas.211291398
Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia
Abstract
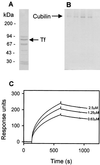
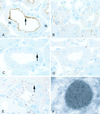
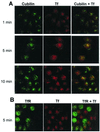
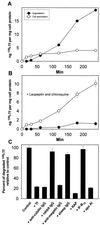
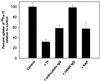
Cubilin is a 460-kDa protein functioning as an endocytic receptor for intrinsic factor vitamin B(12) complex in the intestine and as a receptor for apolipoprotein A1 and albumin reabsorption in the kidney proximal tubules and the yolk sac. In the present study, we report the identification of cubilin as a novel transferrin (Tf) receptor involved in catabolism of Tf. Consistent with a cubilin-mediated endocytosis of Tf in the kidney, lysosomes of human, dog, and mouse renal proximal tubules strongly accumulate Tf, whereas no Tf is detectable in the endocytic apparatus of the renal tubule epithelium of dogs with deficient surface expression of cubilin. As a consequence, these dogs excrete increased amounts of Tf in the urine. Mice with deficient synthesis of megalin, the putative coreceptor colocalizing with cubilin, also excrete high amounts of Tf and fail to internalize Tf in their proximal tubules. However, in contrast to the dogs with the defective cubilin expression, the megalin-deficient mice accumulate Tf on the luminal cubilin-expressing surface of the proximal tubule epithelium. This observation indicates that megalin deficiency causes failure in internalization of the cubilin-ligand complex. The megalin-dependent, cubilin-mediated endocytosis of Tf and the potential of the receptors thereby to facilitate iron uptake were further confirmed by analyzing the uptake of (125)I- and (59)Fe-labeled Tf in cultured yolk sac cells.
Figures

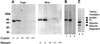
References
-
- de Silva D M, Askwith C C, Kaplan J. Physiol Rev. 1996;76:31–47. - PubMed
-
- Kawabata H, Yang R, Hirama T, Vuong P T, Kawano S, Gombart A F, Koeffler H P. J Biol Chem. 1999;274:20826–20832. - PubMed
-
- Kristiansen M, Graversen J H, Jacobsen C, Sonne O, Hoffman H J, Law S K, Moestrup S K. Nature (London) 2001;409:198–201. - PubMed
-
- Tolosano E, Hirsch E, Patrucco E, Camaschella C, Navone R, Silengo L, Altruda F. Blood. 1999;94:3906–3914. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

