Deamidation of human proteins
- PMID: 11606750
- PMCID: PMC60067
- DOI: 10.1073/pnas.221463198
Deamidation of human proteins
Abstract
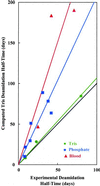
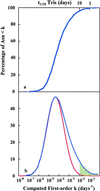
Deamidation of asparaginyl and glutaminyl residues causes time-dependent changes in charge and conformation of peptides and proteins. Quantitative and experimentally verified predictive calculations of the deamidation rates of 1,371 asparaginyl residues in a representative collection of 126 human proteins have been performed. These rates suggest that deamidation is a biologically relevant phenomenon in a remarkably large percentage of human proteins.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

