Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae
- PMID: 11606751
- PMCID: PMC60105
- DOI: 10.1073/pnas.221466798
Gambicin: a novel immune responsive antimicrobial peptide from the malaria vector Anopheles gambiae
Abstract
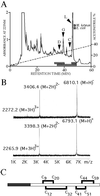
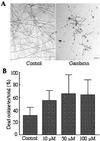
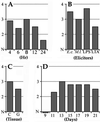
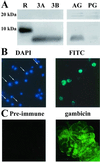
A novel mosquito antimicrobial peptide, gambicin, and the corresponding gene were isolated in parallel through differential display-PCR, an expressed sequence tag (EST) project, and characterization of an antimicrobial activity in a mosquito cell line by reverse-phase chromatography. The 616-bp gambicin ORF encodes an 81-residue protein that is processed and secreted as a 61-aa mature peptide containing eight cysteines engaged in four disulfide bridges. Gambicin lacks sequence homology with other known proteins. Like other Anopheles gambiae antimicrobial peptide genes, gambicin is induced by natural or experimental infection in the midgut, fatbody, and hemocyte-like cell lines. Within the midgut, gambicin is predominantly expressed in the anterior part. Both local and systemic gambicin expression is induced during early and late stages of natural malaria infection. In vitro experiments showed that the 6.8-kDa mature peptide can kill both Gram-positive and Gram-negative bacteria, has a morphogenic effect on a filamentous fungus, and is marginally lethal to Plasmodium berghei ookinetes. An oxidized form of gambicin isolated from the cell line medium was more active against bacteria than the nonoxidized form from the same medium.
Figures


References
-
- Beier J C. Annu Rev Entomol. 1998;43:519–543. - PubMed
-
- Collins F H, Sakai R K, Vernick K D, Paskewitz S, Seeley D C, Miller L H, Collins W E, Campbell C C, Gwadz R W. Science. 1986;234:607–610. - PubMed
-
- Vernick K D, Fujioka H, Seeley D C, Tandler B, Aikawa M, Miller L H. Exp Parasitol. 1995;80:583–595. - PubMed
-
- Ghosh A, Edwards M J, Jacobs-Lorena M. Parasitol Today. 2000;16:196–201. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

