Mechanism of indinavir-induced hyperbilirubinemia
- PMID: 11606755
- PMCID: PMC60112
- DOI: 10.1073/pnas.231140698
Mechanism of indinavir-induced hyperbilirubinemia
Abstract
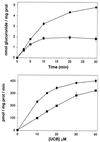
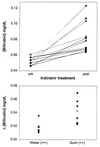
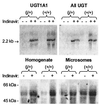
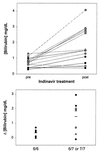
Indinavir is a viral protease inhibitor used for the treatment of HIV infection. Unconjugated hyperbilirubinemia develops in up to 25% of patients receiving indinavir, prompting drug discontinuation and further clinical evaluation in some instances. We postulated that this side-effect is due to indinavir-mediated impairment of bilirubin UDP-glucuronosyltransferase (UGT) activity and would be most pronounced in individuals with reduced hepatic enzyme levels, as occurs in approximately 10% of the population manifesting Gilbert's syndrome. This hypothesis was tested in vitro, in the Gunn rat model of UGT deficiency, and in HIV-infected patients with and without the Gilbert's polymorphism. Indinavir was found to competitively inhibit UGT enzymatic activity (K(I) = 183 microM) while concomitantly inducing hepatic bilirubin UGT mRNA and protein expression. Although oral indinavir increased plasma bilirubin levels in wild-type and heterozygous Gunn rats, the mean rise was significantly greater in the latter group of animals. Similarly, serum bilirubin increased by a mean of 0.34 mg/dl in indinavir-treated HIV patients lacking the Gilbert's polymorphism versus 1.45 mg/dl in those who were either heterozygous or homozygous for the mutant allele. Whereas saquinavir also competitively inhibits UGT activity, this drug has not been associated with hyperbilirubinemia, most likely because of the higher K(I) (360 microM) and substantially lower therapeutic levels as compared with indinavir. Taken together, these findings indicate that elevations in serum-unconjugated bilirubin associated with indinavir treatment result from direct inhibition of bilirubin-conjugating activity.
Figures



Comment in
-
Is "Gilbert's" the culprit in indinavir-induced hyperbilirubinemia?Hepatology. 2002 May;35(5):1269-70. doi: 10.1053/jhep.2002.0351269. Hepatology. 2002. PMID: 11981777 No abstract available.
References
-
- Hammer S M, Squires K E, Hughes M E, Grimes J M, Demeter L M, Currier J S, Eron J J, Feinberg J E, Balfour H H, Deyton L R, et al. N Engl J Med. 1997;337:725–733. - PubMed
-
- Roy M G, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, et al. N Engl J Med. 1997;337:734–739. - PubMed
-
- Stein D S, Fish D G, Bilello J A, Preston S L, Martineau G L, Drusano G L. AIDS. 1996;10:485–492. - PubMed
-
- Kaul D R, Cinti S K, Carver P L, Kazanjian P H. Pharmacotherapy. 1999;19:281–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

