Identification of sites for exponential translation in living dendrites
- PMID: 11606784
- PMCID: PMC60820
- DOI: 10.1073/pnas.231485698
Identification of sites for exponential translation in living dendrites
Abstract
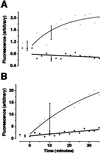
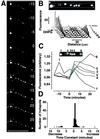
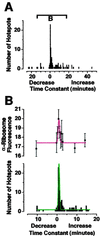
Neuronal processes contain mRNAs and membrane structures, and some forms of synaptic plasticity seem to require protein synthesis in dendrites of hippocampal neurons. To quantitate dendritic protein synthesis, we used multiphoton microscopy of green fluorescent protein synthesized in living isolated dendrites. Transfection of dendrites with mRNA encoding green fluorescent protein resulted in fluorescence that exponentially increased on stimulation with a glutamate receptor agonist; a reaction attenuated by the translation inhibitors anisomycin and emetine. Comparable experiments on whole neurons revealed that (RS)-3,5-dihydroxy-phenylglycine 0.5 H(2)O (DHPG)-stimulated fluorescence was linear in cell bodies relative to the exponential increase seen in dendrites. Detailed spatial analysis of the subdendritic distribution of fluorescence revealed "hotspots," sites of dendritic translation that were spatially stable. However, detailed temporal analysis of these hotspots revealed heterogeneous rates of translation. A double-label protocol counterstaining for ribosomes indicated that sites of "fastest" translation correlated with increased ribosome density, consistent with ribosome subunit assembly for initiation, the first step of translation. We propose that dendrites have specific sites specialized for fast translation.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

