An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans
- PMID: 11641215
- PMCID: PMC2430591
- DOI: 10.1242/dev.128.20.3899
An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans
Abstract
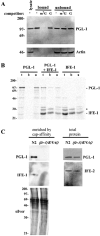
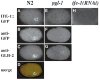
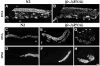
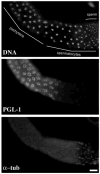
Control of gene expression at the translational level is crucial for many developmental processes. The mRNA cap-binding protein, eIF4E, is a key player in regulation of translation initiation; appropriate levels of eIF4E are essential for normal cell-cycle regulation and tissue differentiation. The observation that eIF4E levels are elevated during gametogenesis in several organisms suggests that eIF4E might have a specific role in gamete formation as well. We show that one of the five isoforms of C. elegans eIF4E, IFE-1, is enriched in the germline and is a component of germ granules (P granules). The association of IFE-1 with P granules requires the P-granule protein PGL-1. In vitro PGL-1 interacts directly with IFE-1, but not with the other four isoforms of eIF4E. Analysis of animals depleted of IFE-1 by RNAi shows that IFE-1 is required for spermatogenesis, specifically for efficient progression through the meiotic divisions and for the production of functional sperm, in both hermaphrodites and males. The requirement for IFE-1 is highly sensitive to temperature. IFE-1 is not required for oogenesis, as ife-1(RNAi) hermaphrodites produce viable progeny when normal sperm are supplied. Consistent with a primary role in spermatogenesis, ife-1 mRNA levels are highest in regions of the gonad undergoing spermatogenesis. Our results suggest that C. elegans spermatogenesis requires either this specific isoform of eIF4E or an elevated level of eIF4E.
Figures
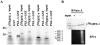

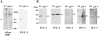


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

