Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA
- PMID: 11641276
- PMCID: PMC312821
- DOI: 10.1101/gad.915701
Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA
Abstract
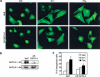
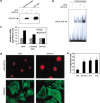
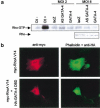
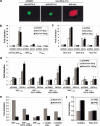
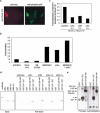
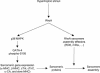
Rho-like GTPases play a pivotal role in the orchestration of changes in the actin cytoskeleton in response to receptor stimulation, and have been implicated in transcriptional activation, cell growth regulation, and oncogenic transformation. Recently, a role for RhoA in the regulation of cardiac contractility and hypertrophic cardiomyocyte growth has been suggested but the mechanisms underlying RhoA function in the heart remain undefined. We now report that transcription factor GATA-4, a key regulator of cardiac genes, is a nuclear mediator of RhoA signaling and is involved in the control of sarcomere assembly in cardiomyocytes. Both RhoA and GATA-4 are essential for sarcomeric reorganization in response to hypertrophic growth stimuli and overexpression of either protein is sufficient to induce sarcomeric reorganization. Consistent with convergence of RhoA and GATA signaling, RhoA potentiates the transcriptional activity of GATA-4 via a p38 MAPK-dependent pathway that phosphorylates GATA-4 activation domains and GATA binding sites mediate RhoA activation of target cardiac promoters. Moreover, a dominant-negative GATA-4 protein abolishes RhoA-induced sarcomere reorganization. The identification of transcription factor GATA-4 as a RhoA mediator in sarcomere reorganization and cardiac gene regulation provides a link between RhoA effects on transcription and cell remodeling.
Figures




References
-
- Acuto O, Cantrell D. T cell activation and the cytoskeleton. Annu Rev Immunol. 2000;18:165–184. - PubMed
-
- Andreka P, Zang J, Dougherty C, Slepak TI, Webster KA, Bishopric NH. Cytoprotection by Jun kinase during nitric oxide-induced cardiac myocyte apoptosis. Circ Res. 2001;88:305–312. - PubMed
-
- Aoki H, Izumo S, Sadoshima J. Angiotensin II activates RhoA in cardiac myocytes: A critical role of RhoA in angiotensin II-induced premyofibril formation. Circ Res. 1998;82:666–676. - PubMed
-
- Bar-Sagi D, Hall A. Ras and Rho GTPases: A family reunion. Cell. 2000;103:227–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
