Deletion of lolB, encoding an outer membrane lipoprotein, is lethal for Escherichia coli and causes accumulation of lipoprotein localization intermediates in the periplasm
- PMID: 11673422
- PMCID: PMC95483
- DOI: 10.1128/JB.183.22.6538-6542.2001
Deletion of lolB, encoding an outer membrane lipoprotein, is lethal for Escherichia coli and causes accumulation of lipoprotein localization intermediates in the periplasm
Abstract
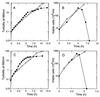
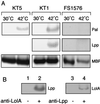
Outer membrane lipoproteins of Escherichia coli are released from the inner membrane upon the formation of a complex with a periplasmic chaperone, LolA, followed by localization to the outer membrane. In vitro biochemical analyses revealed that the localization of lipoproteins to the outer membrane generally requires an outer membrane lipoprotein, LolB, and occurs via transient formation of a LolB-lipoprotein complex. On the other hand, a mutant carrying the chromosomal lolB gene under the control of the lac promoter-operator grew normally in the absence of LolB induction if the mutant did not possess the major outer membrane lipoprotein Lpp, suggesting that LolB is only important for the localization of Lpp in vivo. To examine the in vivo function of LolB, we constructed a chromosomal lolB null mutant harboring a temperature-sensitive helper plasmid carrying the lolB gene. At a nonpermissive temperature, depletion of the LolB protein due to loss of the lolB gene caused cessation of growth and a decrease in the number of viable cells irrespective of the presence or absence of Lpp. LolB-depleted cells accumulated the LolA-lipoprotein complex in the periplasm and the mature form of lipoproteins in the inner membrane. Taken together, these results indicate that LolB is the first example of an essential lipoprotein for E. coli and that its depletion inhibits the upstream reactions of lipoprotein trafficking.
Figures


References
-
- Armstrong K A, Acosta R, Ledner E, Machida Y, Pancotto M, McCormick M, Ohtsubo H, Ohtsubo E. A 37 × 103 molecular weight plasmid-encoded protein is required for replication and copy number control in the plasmid pSC101 and its temperature-sensitive derivative pHS1. J Mol Biol. 1984;175:331–348. - PubMed
-
- Cabello F, Timmis K, Cohen S N. Replication control in a composite plasmid constructed by in vitro linkage of two distinct replicons. Nature. 1976;259:285–290. - PubMed
-
- Campos N, Rodriguez-Concepcion M, Sauret-Gueto S, Gallego F, Lois L M, Boronat A. Escherichia coli engineered to synthesize isopentenyl diphosphate and dimethylallyl diphosphate from mevalonate: a novel system for the genetic analysis of the 2-C-methyl-d-erythritol 4-phosphate pathway for isoprenoid biosynthesis. Biochem J. 2001;353:59–67. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

