Vibrio fischeri outer membrane protein OmpU plays a role in normal symbiotic colonization
- PMID: 11673429
- PMCID: PMC95490
- DOI: 10.1128/JB.183.22.6590-6597.2001
Vibrio fischeri outer membrane protein OmpU plays a role in normal symbiotic colonization
Abstract
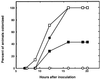
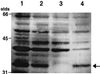
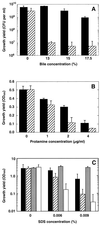
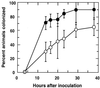
The nascent light-emitting organ of newly hatched juveniles of the Hawaiian sepiolid squid Euprymna scolopes is specifically colonized by cells of Vibrio fischeri that are obtained from the ambient seawater. The mechanisms that promote this specific, cooperative colonization are likely to require a number of bacterial and host-derived factors and activities, only some of which have been described to date. A characteristic of many host-pathogen associations is the presence of bacterial mechanisms that allow attachment to specific tissues. These mechanisms have been well characterized and often involve bacterial fimbriae or outer membrane proteins (OMPs) that act as adhesins, the expression of which has been linked to virulence regulators such as ToxR in Vibrio cholerae. Analogous or even homologous mechanisms are probably operative in the initiation and persistence of cooperative bacterial associations, although considerably less is known about them. We report the presence in V. fischeri of ompU, a gene encoding a 32.5-kDa protein homolog of two other OMPs, OmpU of V. cholerae (50.8% amino acid sequence identity) and OmpL of Photobacterium profundum (45.5% identity). A null mutation introduced into the V. fischeri ompU resulted in the loss of an OMP with an estimated molecular mass of about 34 kDa; genetic complementation of the mutant strain with a DNA fragment containing only the ompU gene restored the production of this protein. The expression of the V. fischeri OmpU was not significantly affected by either (i) iron or phosphate limitation or (ii) a mutation that renders V. fischeri defective in the synthesis of a homolog of the OMP-regulatory protein ToxR. The ompU mutant grew normally in complex nutrient media but was more susceptible to growth inhibition in the presence of either anionic detergents or the antimicrobial peptide protamine sulfate. Interestingly, colonization experiments showed that the ompU null mutant initiated a symbiotic association with juvenile light organ tissue with only about 60% of the effectiveness of the parent strain. When colonization did occur, it proceeded more slowly and resulted in an approximately fourfold-smaller bacterial population. Surprisingly, there was no evidence that in a mixed infection with its parent, the ompU-defective strain had a competitive disadvantage, suggesting that the presence of the parent strain provided a shared compensatory activity. Thus, the OmpU protein appears to play a role in the normal process by which V. fischeri initiates its colonization of the nascent light organ of juvenile squids.
Figures



References
-
- Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980.
-
- Douglas A E. Symbiotic interactions. Oxford, England: Oxford Science Publications; 1994.
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

