Escherichia coli division inhibitor MinCD blocks septation by preventing Z-ring formation
- PMID: 11673433
- PMCID: PMC95494
- DOI: 10.1128/JB.183.22.6630-6635.2001
Escherichia coli division inhibitor MinCD blocks septation by preventing Z-ring formation
Abstract
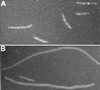
The min system spatially regulates division through the topological regulation of MinCD, an inhibitor of cell division. MinCD was previously shown to inhibit division by preventing assembly of the Z ring (E. Bi and J. Lutkenhaus, J. Bacteriol. 175:1118-1125, 1993); however, this was questioned in a recent report (S. S. Justice, J. Garcia-Lara, and L. I. Rothfield, Mol. Microbiol. 37:410-423, 2000) which indicated that MinCD acted after Z-ring formation and prevented the recruitment of FtsA to the Z ring. This discrepancy was due in part to alternative fixation conditions. We have therefore reinvestigated the action of MinCD and avoided fixation by using green fluorescent protein (GFP) fusions to division proteins. MinCD prevented the localization of both FtsZ-GFP and ZipA-GFP, consistent with it preventing Z-ring assembly. Consistent with a direct interaction between FtsZ and the MinCD inhibitor, we find that increased FtsZ, but not FtsA, suppresses MinCD-induced lethality. Furthermore, strains carrying various alleles of ftsZ, selected on the basis of resistance to the inhibitor SulA, displayed variable resistance to MinCD. These results are consistent with FtsZ as the target of MinCD and confirm that this inhibitor prevents Z-ring assembly.
Figures


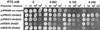
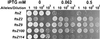
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

