Accumulation of cholera toxin and GM1 ganglioside in the early endosome of Niemann-Pick C1-deficient cells
- PMID: 11675488
- PMCID: PMC60064
- DOI: 10.1073/pnas.221181998
Accumulation of cholera toxin and GM1 ganglioside in the early endosome of Niemann-Pick C1-deficient cells
Abstract
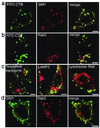
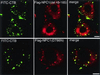
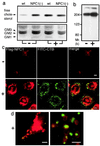
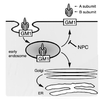
We investigated intracellular trafficking of GM1 ganglioside in Niemann-Pick C1 (NPC1)-deficient Chinese hamster ovary cells [NPC1(-) cells] by using cholera toxin (CT) as a probe. Both the holotoxin and the B subunit (CTB) accumulated in GM1-enriched intracellular vesicles of NPC1(-) cells. CTB-labeled vesicles contained the early endosome marker Rab5 but not lysosome-associated membrane protein 2 and were not labeled with either Texas red-transferrin or Lysotracker, indicating that they represent early endosomes. Similarly, CT accumulated in intracellular vesicles of human NPC fibroblasts that contained both Rab5 and early endosomal antigen 1. CTB accumulation in NPC1(-) cells was abolished by expression of wild-type NPC1 but not by mutant proteins with a mutation either in the NPC domain or the sterol-sensing domain. A part of these mutant NPC1 proteins expressed in NPC1(-) cells was localized on CTB-labeled vesicles. U18666A treatment of "knock in" cells [NPC1(-) cells that stably expressed wild-type NPC1] caused CTB accumulation similar to that in NPC1(-) cells, and a part of wild-type NPC1was localized on CTB-labeled vesicles in drug-treated cells. Finally, CT tracer experiments in NPC1(-) cells revealed retarded excretion of internalized toxin into the culture medium and an increase in the intracellular release of A subunits. In accordance with the latter result, CT was more effective in stimulating cAMP formation in NPC1(-) than in wild-type cells. These results suggest that transport of CT/GM1 complexes from the early endosome to the plasma membrane depends on the function of NPC1, whereas transport to the Golgi apparatus/endoplasmic reticulum does not.
Figures



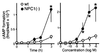
References
-
- Carstea E D, Morris J A, Coleman K G, Loftus S K, Zhang D, Cummings C, Gu J, Rosenfeld M A, Pavan W J, et al. Science. 1997;277:228–231. - PubMed
-
- Loftus S K, Morris J A, Carstea E D, Gu J Z, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld M A, et al. Science. 1997;277:232–235. - PubMed
-
- Davies J P, Ioannou Y A. J Biol Chem. 2000;275:24367–24374. - PubMed
-
- Ioannou Y A. Mol Genet Metab. 2000;71:175–181. - PubMed
-
- Neufeld E B, Wastney M, Patel S, Suresh S, Cooney A M, Dwyer N K, Roff C F, Ohno K, Morris J A, et al. J Biol Chem. 1999;274:9627–9635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

