Transient uptake of serotonin by newborn olfactory projection neurons
- PMID: 11675504
- PMCID: PMC60122
- DOI: 10.1073/pnas.231471298
Transient uptake of serotonin by newborn olfactory projection neurons
Abstract
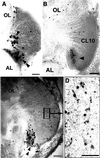
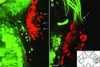
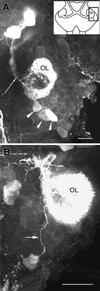
A life-long turnover of sensory and interneuronal populations has been documented in the olfactory pathways of both vertebrates and invertebrates, creating a situation where the axons of new afferent and interneuronal populations must insert into a highly specialized glomerular neuropil. A dense serotonergic innervation of the primary olfactory processing areas where these neurons synapse also is a consistent feature across species. Prior studies in lobsters have shown that serotonin promotes the branching of olfactory projection neurons. This paper presents evidence that serotonin also regulates the proliferation and survival of projection neurons in lobsters, and that the serotonergic effects are associated with a transient uptake of serotonin into newborn neurons.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

