Multiple dose study of interactions between artesunate and artemisinin in healthy volunteers
- PMID: 11678781
- PMCID: PMC2014589
- DOI: 10.1046/j.0306-5251.2001.01461.x
Multiple dose study of interactions between artesunate and artemisinin in healthy volunteers
Abstract
Aims: To investigate whether coadministration of the antimalarials artesunate and artemisinin alters the clearance of either drug.
Methods: Ten healthy Vietnamese males (Group AS) were randomized to receive a single dose of 100 mg oral artesunate (pro-drug of dihydroartemisinin) on day -5 and then once daily for 5 consecutive days (days 1-5). Oral artemisinin (500 mg) was coadministered on days 1 and 5. Another 10 subjects (Group AM) were given 500 mg oral artemisinin on day -5 and then further doses on days 1-5. Artesunate 100 mg was given on days 1 and 5. Artemisinin and dihydroartemisinin plasma concentrations on days -5, 1 and 5 were quantified by h.p.l.c. with on-line postcolumn derivatization and u.v. detection.
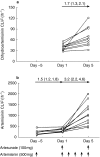
Results: In Group AS, dihydroartemisinin oral clearance values (mean (95% CI)) were similar on day 1 (32 (22, 47)) l h(-1) and day 5 (38 (28, 51)) l h(-1) of daily artesunate administration but these mean values were approximately three fold higher compared with day -5 after a single dose (95 (56, 159)). In this group, artemisinin oral clearance increased from 196 (165, 232) l h(-1) on day 1-315 (241, 410) l h(-1) on day 5. In Group AM, dihydroartemisinin oral clearance on day 1 was 39 (34, 46) l h(-1) and increased 1.6 fold to 64 (48, 85) l h(-1) on day 5. In this group, artemisinin oral clearance increased sequentially (1.5 and 4.7 fold, respectively) from 207 (151, 285) l h(-1) on day -5-308 (257, 368) l h(-1) on day 1 and to 981 (678, 1420) l h(-1) on day 5. The increase in artemisinin oral clearance between days -5 and 1 (in the absence of artesunate) was similar to that between days 1 and 5 in Group AS subjects who took daily artesunate. Dihydroartemisinin was not a significant metabolite of artemisinin.
Conclusions: Artesunate (dihydroartemisinin) did not alter the elimination of artemisinin. However, dihydroartemisinin elimination was inhibited by artemisinin. Artemisinin induced its own elimination even 5 days after a single oral dose. There was no evidence for the formation of dihydroartemisinin from artemisinin.
Figures


Similar articles
-
Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers.Am J Trop Med Hyg. 1997 Jan;56(1):17-23. doi: 10.4269/ajtmh.1997.56.17. Am J Trop Med Hyg. 1997. PMID: 9063354 Clinical Trial.
-
Pharmacokinetics of artesunate following oral and rectal administration in healthy Sudanese volunteers.Trop Doct. 2004 Jul;34(3):132-5. doi: 10.1177/004947550403400302. Trop Doct. 2004. PMID: 15267037 Clinical Trial.
-
Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients.Trans R Soc Trop Med Hyg. 1996 Jan-Feb;90(1):61-5. doi: 10.1016/s0035-9203(96)90480-0. Trans R Soc Trop Med Hyg. 1996. PMID: 8730315 Clinical Trial.
-
Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration.Malar J. 2011 Sep 13;10:263. doi: 10.1186/1475-2875-10-263. Malar J. 2011. PMID: 21914160 Free PMC article. Review.
-
Rectal administration of artemisinin derivatives for the treatment of malaria.JAMA. 2007 Jun 6;297(21):2381-90. doi: 10.1001/jama.297.21.2381. JAMA. 2007. PMID: 17551131 Review.
Cited by
-
Artemisinin and CYP2A6 activity in healthy subjects.Eur J Clin Pharmacol. 2008 Mar;64(3):283-92. doi: 10.1007/s00228-007-0406-1. Epub 2007 Dec 7. Eur J Clin Pharmacol. 2008. PMID: 18064444 Clinical Trial.
-
Population pharmacokinetics of artesunate and dihydroartemisinin following single- and multiple-dosing of oral artesunate in healthy subjects.Malar J. 2009 Dec 18;8:304. doi: 10.1186/1475-2875-8-304. Malar J. 2009. PMID: 20021657 Free PMC article. Clinical Trial.
-
Disposition of artesunate and dihydroartemisinin after administration of artesunate suppositories in children from Papua New Guinea with uncomplicated malaria.Antimicrob Agents Chemother. 2004 Aug;48(8):2966-72. doi: 10.1128/AAC.48.8.2966-2972.2004. Antimicrob Agents Chemother. 2004. PMID: 15273107 Free PMC article. Clinical Trial.
-
Pharmacokinetics and Toxicokinetics of Artemisinin-Hydroxychloroquine Sulfate Tablets in Rats and Dogs.Evid Based Complement Alternat Med. 2021 Oct 21;2021:6830459. doi: 10.1155/2021/6830459. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34721641 Free PMC article.
-
Orally formulated artemisinin in healthy fasting Vietnamese male subjects: a randomized, four-sequence, open-label, pharmacokinetic crossover study.Clin Ther. 2011 May;33(5):644-54. doi: 10.1016/j.clinthera.2011.04.017. Clin Ther. 2011. PMID: 21665048 Free PMC article. Clinical Trial.
References
-
- Arnold K, Hien TT, Chinh NT, Phu NH, Mai PP. A randomised comparative study of artemisinin (qinghaosu) suppositories and oral quinine in acute falciparum malaria. Trans Roy Soc Trop Med Hyg. 1990;84:499–502. - PubMed
-
- Cao XT, Bethell DB, Pham TP, et al. Comparison of artemisinin suppositories, intramuscular artesunate and intravenous quinine for the treatment of severe childhood malaria. Trans Roy Soc Trop Med Hyg. 1997;91:335–342. - PubMed
-
- Vinh H, Huong NN, Ha TTB, et al. Severe and complicated malaria treated with artemisinin, artesunate or artemether in Viet Nam. Trans Roy Soc Trop Med Hyg. 1997;91:465–467. - PubMed
-
- Hien TT, White NJ. Qinghaosu. Lancet. 1993;341:603–608. - PubMed
-
- Sy DN, Hoan DB, Dung NP, et al. Treatment of malaria in Vietnam with oral artemisinin. Am J Trop Med Hyg. 1993;48:398–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

