Differentiation of antineutrophil nuclear antibodies in inflammatory bowel and autoimmune liver diseases from antineutrophil cytoplasmic antibodies (p-ANCA) using immunofluorescence microscopy
- PMID: 11678897
- PMCID: PMC1906166
- DOI: 10.1046/j.1365-2249.2001.01649.x
Differentiation of antineutrophil nuclear antibodies in inflammatory bowel and autoimmune liver diseases from antineutrophil cytoplasmic antibodies (p-ANCA) using immunofluorescence microscopy
Abstract
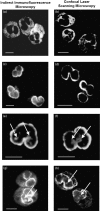
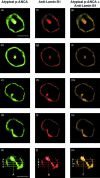
Perinuclear antineutrophil cytoplasmic antibodies (p-ANCA) directed against cytoplasmic proteins of neutrophils have been studied extensively in patients with systemic vasculitides. Recent data indicate that antineutrophil antibodies in sera from patients with chronic inflammatory bowel diseases (IBD) or autoimmune liver disorders, currently called 'atypical p-ANCA', recognize a nuclear target antigen, rendering the term 'ANCA' inaccurate. Specific microscopic criteria to distinguish atypical p-ANCA from p-ANCA are lacking. We used planar and confocal laser scanning indirect immunofluorescence microscopy to examine the labelling characteristics of ethanol-, methanol- and formaldehyde-fixed neutrophils by antineutrophil antibodies in 153 serum samples from patients with IBD, autoimmune liver disorders, systemic vasculitides or healthy blood donors. On ethanol- or methanol-fixed neutrophils, multiple intranuclear fluorescent foci together with either a rim-like peripheral nuclear staining ('type A') or a combined cytoplasmic and peripheral nuclear staining ('type B') was noted exclusively with atypical p-ANCA in sera from patients with IBD or autoimmune liver disorders. Intranuclear foci, which probably corresponded to invaginations of the nuclear envelope, were not labelled by p-ANCA from patients with microscopic polyangiitis or cytoplasmic ANCA (c-ANCA) from patients with Wegener's granulomatosis. On formaldehyde-fixed neutrophils, atypical p-ANCA gave a fine rim-like staining of the nuclear periphery, whereas ANCA diffusely labelled the cytoplasm. To distinguish reliably between the patterns produced by atypical p-ANCA or p-ANCA, particularly p-ANCA, careful indirect immunofluorescence microscopy on ethanol- as well as on formaldehyde-fixed neutrophils is necessary, with particular emphasis on the presence of multiple intranuclear fluorescent foci.
Figures


Similar articles
-
Atypical antineutrophil cytoplasmic antibodies with perinuclear fluorescence in chronic inflammatory bowel diseases and hepatobiliary disorders colocalize with nuclear lamina proteins.Hepatology. 1998 Aug;28(2):332-40. doi: 10.1002/hep.510280207. Hepatology. 1998. PMID: 9695994
-
Comparative evaluation of unfixed and fixed human neutrophils for determination of antineutrophil cytoplasmic antibodies by indirect immunofluorescence.J Clin Pathol. 1997 Aug;50(8):677-80. doi: 10.1136/jcp.50.8.677. J Clin Pathol. 1997. PMID: 9301553 Free PMC article. Clinical Trial.
-
Antineutrophil nuclear antibodies (ANNA) in primary biliary cirrhosis: their prevalence and antigen specificity.Z Gastroenterol. 1997 Feb;35(2):113-21. Z Gastroenterol. 1997. PMID: 9066101
-
Antineutrophil cytoplasmic antibodies (ANCA).Autoimmunity. 2005 Feb;38(1):93-103. doi: 10.1080/08916930400022673. Autoimmunity. 2005. PMID: 15804710 Review.
-
Atypical autoantigen targets of perinuclear antineutrophil cytoplasm antibodies (P-ANCA): specificity and clinical associations.J Autoimmun. 1993 Apr;6(2):185-95. doi: 10.1006/jaut.1993.1016. J Autoimmun. 1993. PMID: 8388691 Review.
Cited by
-
Antineutrophil cytoplasmic antibody profiles differ according to type of primary sclerosing cholangitis and autoimmune hepatitis.Clinics (Sao Paulo). 2021 Feb 5;76:e2228. doi: 10.6061/clinics/2021/e2228. eCollection 2021. Clinics (Sao Paulo). 2021. PMID: 33567045 Free PMC article.
-
Automated interpretation of ANCA patterns - a new approach in the serology of ANCA-associated vasculitis.Arthritis Res Ther. 2012 Dec 14;14(6):R271. doi: 10.1186/ar4119. Arthritis Res Ther. 2012. PMID: 23241527 Free PMC article.
-
Autoantibodies in primary sclerosing cholangitis.World J Gastroenterol. 2008 Jun 28;14(24):3781-91. doi: 10.3748/wjg.14.3781. World J Gastroenterol. 2008. PMID: 18609700 Free PMC article. Review.
-
Utility of serological markers in inflammatory bowel diseases: gadget or magic?World J Gastroenterol. 2007 Apr 14;13(14):2028-36. doi: 10.3748/wjg.v13.i14.2028. World J Gastroenterol. 2007. PMID: 17465443 Free PMC article. Review.
-
IgA class antineutrophil cytoplasmic antibodies in primary sclerosing cholangitis and autoimmune hepatitis.Clin Exp Immunol. 2003 Aug;133(2):283-9. doi: 10.1046/j.1365-2249.2003.02195.x. Clin Exp Immunol. 2003. PMID: 12869036 Free PMC article.
References
-
- Woude FJ, Lobatto S, Permin H, et al. Autoantibodies against neutrophils and monocytes: tools for diagnosis and marker of disease activity in Wegener's granulomatosis. Lancet. 1985;1:425–9. - PubMed
-
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–7. - PubMed
-
- Duerr RH, Targan SR, Landers CJ, et al. Neutrophil cytoplasmic antibodies: a link between primary sclerosing cholangitis and ulcerative colitis. Gastroenterology. 1991;100:1385–91. - PubMed
-
- Oudkerk-Pool M, Ellerbroek PM, Ridwan BU, et al. Serum antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease are mainly associated with ulcerative colitis. A correlation study between perinuclear antineutrophil cytoplasmic autoantibodies and clinical parameters, medical and surgical treatment. Gut. 1993;34:46–60. - PMC - PubMed
-
- Mulder AHL, Horst G, Haagsma EB, Kleibeuker JH, Kallenberg CGM. Antineutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993;17:411–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

