Detection of the isiA gene across cyanobacterial strains: potential for probing iron deficiency
- PMID: 11679352
- PMCID: PMC93297
- DOI: 10.1128/AEM.67.11.5247-5253.2001
Detection of the isiA gene across cyanobacterial strains: potential for probing iron deficiency
Abstract
The use of isiA expression to monitor the iron status of cyanobacteria was investigated. Studies of laboratory cultures of the cyanobacterium Synechocystis sp. strain PCC 6803 showed that isiA expression is dependent on the organism's response to iron deficiency; isiA expression starts as soon as a decline in the rate of growth begins. isiA expression is switched on at concentrations of iron citrate of less than 0.7 microM. A PCR method was developed for the specific amplification of the iron-regulated isiA gene from a variety of cyanobacteria. After we developed degenerate primers, 15 new internal isiA fragments (840 bp) were amplified, cloned, and sequenced from strains obtained from algal collections, from new isolates, and from enriched field samples. Furthermore, isiA expression could be detected by means of reverse transcription-PCR when enriched field samples were exposed to restricted iron availability. These results imply that determining the level of iron-regulated isiA expression can serve to indicate iron deficiency in cyanobacterial samples of differing origins from the field.
Figures



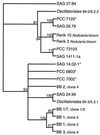
 ) and from the present study of different cyanobacterial strains, clones from field samples, and enrichment cultures. Bootstrap values were calculated after 1,000 replications; only branchings with values above 50% are shown.
) and from the present study of different cyanobacterial strains, clones from field samples, and enrichment cultures. Bootstrap values were calculated after 1,000 replications; only branchings with values above 50% are shown.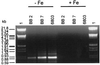
Similar articles
-
The iron-regulated isiA gene of Fischerella muscicola strain PCC 73103 is linked to a likewise regulated gene encoding a Pcb-like chlorophyll-binding protein.FEMS Microbiol Lett. 2001 Apr 1;197(1):123-9. doi: 10.1111/j.1574-6968.2001.tb10593.x. FEMS Microbiol Lett. 2001. PMID: 11287157
-
Expression of the isiA gene is essential for the survival of the cyanobacterium Synechococcus sp. PCC 7942 by protecting photosystem II from excess light under iron limitation.Mol Microbiol. 1999 Apr;32(1):123-9. doi: 10.1046/j.1365-2958.1999.01332.x. Mol Microbiol. 1999. PMID: 10216865
-
An iron stress operon involved in photosynthetic electron transport in the marine cyanobacterium Synechococcus sp. PCC 7002.J Gen Microbiol. 1992 Aug;138 Pt 8:1613-21. doi: 10.1099/00221287-138-8-1613. J Gen Microbiol. 1992. PMID: 1527503
-
Structure and functional role of supercomplexes of IsiA and Photosystem I in cyanobacterial photosynthesis.FEBS Lett. 2005 Jun 13;579(15):3253-7. doi: 10.1016/j.febslet.2005.03.051. Epub 2005 Apr 7. FEBS Lett. 2005. PMID: 15943969 Review.
-
Regulation and Functional Complexity of the Chlorophyll-Binding Protein IsiA.Front Microbiol. 2021 Nov 17;12:774107. doi: 10.3389/fmicb.2021.774107. eCollection 2021. Front Microbiol. 2021. PMID: 34867913 Free PMC article. Review.
Cited by
-
Detection of Prochlorothrix in brackish waters by specific amplification of pcb genes.Appl Environ Microbiol. 2003 Oct;69(10):6243-9. doi: 10.1128/AEM.69.10.6243-6249.2003. Appl Environ Microbiol. 2003. PMID: 14532086 Free PMC article.
-
Biogeography of Cyanobacterial isiA Genes and Their Link to Iron Availability in the Ocean.Front Microbiol. 2019 Apr 4;10:650. doi: 10.3389/fmicb.2019.00650. eCollection 2019. Front Microbiol. 2019. PMID: 31024472 Free PMC article.
-
Biogeography of photosynthetic light-harvesting genes in marine phytoplankton.PLoS One. 2009;4(2):e4601. doi: 10.1371/journal.pone.0004601. Epub 2009 Feb 25. PLoS One. 2009. PMID: 19240807 Free PMC article.
-
Function of the IsiA pigment-protein complex in vivo.Photosynth Res. 2019 Sep;141(3):343-353. doi: 10.1007/s11120-019-00638-5. Epub 2019 Mar 30. Photosynth Res. 2019. PMID: 30929163
-
Coupling of excitation energy to photochemistry in natural marine phytoplankton communities under iron stress.Proc Natl Acad Sci U S A. 2025 Aug 5;122(31):e2511916122. doi: 10.1073/pnas.2511916122. Epub 2025 Jul 29. Proc Natl Acad Sci U S A. 2025. PMID: 40729384
References
-
- Abraham E R, Law C S, Boyd P W, Lavender S J, Maldonado M T, Bowie A R. Importance of stirring in the development of an iron-fertilized phytoplankton bloom. Nature. 2000;407:727–730. - PubMed
-
- Brand L E. Minimum iron requirements of marine phytoplankton and the implications for the biogeochemical control of new production. Limnol Oceanogr. 1991;36:1756–1771.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical

