Structure-activity relationships of diadenosine polyphosphates (Ap(n)As), adenosine polyphospho guanosines (Ap(n)Gs) and guanosine polyphospho guanosines (Gp(n)Gs) at P2 receptors in the rat mesenteric arterial bed
- PMID: 11682456
- PMCID: PMC1573034
- DOI: 10.1038/sj.bjp.0704341
Structure-activity relationships of diadenosine polyphosphates (Ap(n)As), adenosine polyphospho guanosines (Ap(n)Gs) and guanosine polyphospho guanosines (Gp(n)Gs) at P2 receptors in the rat mesenteric arterial bed
Abstract
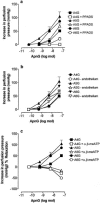
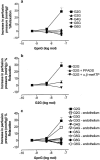
1. Vascular effects of diadenosine polyphosphates (Ap(n)As), adenosine polyphospho guanosines (Ap(n)Gs) and guanosine polyphospho guanosines (Gp(n)Gs), novel families of naturally-occurring signalling molecules, were investigated in methoxamine preconstricted rat isolated perfused mesenteric arterial beds. 2. Three different types of response were elicited by Ap(n)As and Ap(n)Gs. Those with a short polyphosphate chain (n=2 - 3) elicited vasorelaxation. Ap(3)A was more potent than Ap(2)A, and both were more potent than the corresponding Ap(n)G. Relaxations to Ap(3)A and Ap(3)G, but not to Ap(2)A and Ap(2)G, were blocked by endothelium removal and pyridoxalphosphate-6-azophenyl-2',4'-disulphonic acid (PPADS), a P2 receptor antagonist. 3. Longer polyphosphate chain Ap(n)As and Ap(n)Gs (n=4 - 6) elicited dose-dependent vasoconstriction followed by prolonged vasorelaxation, with a potency order for both types of response of Ap(5)A> or =Ap(6)A>Ap(4)A. A similar order and potency was observed for Ap(n)Gs. Contractions and prolonged relaxations were blocked by PPADS and P2X(1) receptor desensitization with alpha,beta-methylene ATP (alpha,beta-meATP), and were largely endothelium-independent. 4. In the presence of alpha,beta-meATP rapid relaxations to contractile Ap(n)As and Ap(n)Gs (n=4 - 6) were revealed. 5. Gp(n)Gs were virtually inactive, except for Gp(2)G which elicited vasoconstriction via PPADS- and alpha,beta-meATP-sensitive smooth muscle P2X(1)-like receptors. 6. These data show that, as with Ap(n)As, the length of the polyphosphate chain (n) is an important determinant of the activity of Ap(n)Gs at P2 receptors in the rat mesenteric arterial bed. When the chain is short (n=2 - 3) the purines elicit rapid vasorelaxation, which for Ap(3)A and Ap(3)G is mediated via endothelial P2Y(1)-like receptors. When the chain is long (n=4 - 6) Ap(n)As and Ap(n)Gs elicit vasoconstriction via P2X(1)-like receptors, followed by prolonged endothelium-independent vasorelaxation. Rapid relaxation to contractile dinucleotides (n=4 - 6) is revealed by block of vasoconstriction. Regarding the purine moiety, one adenine is crucial and sufficient for vasoactivity as Gp(n)Gs were largely inactive, and Ap(n)As and Ap(n)Gs approximately equipotent.
Figures








Similar articles
-
The involvement of smooth muscle P2X receptors in the prolonged vasorelaxation response to purine nucleotides in the rat mesenteric arterial bed.Br J Pharmacol. 2002 Apr;135(8):1988-94. doi: 10.1038/sj.bjp.0704663. Br J Pharmacol. 2002. PMID: 11959802 Free PMC article.
-
Effects of diadenosine polyphosphates (Ap(n)As) and adenosine polyphospho guanosines (Ap(n)Gs) on rat mesenteric artery P2X receptor ion channels.Br J Pharmacol. 2000 Jan;129(1):124-30. doi: 10.1038/sj.bjp.0702993. Br J Pharmacol. 2000. PMID: 10694211 Free PMC article.
-
Relative contribution of P2U- and P2Y-purinoceptors to endothelium-dependent vasodilatation in the golden hamster isolated mesenteric arterial bed.Br J Pharmacol. 1996 Apr;117(8):1797-802. doi: 10.1111/j.1476-5381.1996.tb15357.x. Br J Pharmacol. 1996. PMID: 8732294 Free PMC article.
-
Mesenteric and renal vascular effects of diadenosine polyphosphates (APnA).Cardiovasc Res. 2002 Oct;56(1):22-32. doi: 10.1016/s0008-6363(02)00533-3. Cardiovasc Res. 2002. PMID: 12237163 Review.
-
Endothelial modulation of agonist-induced vasoconstriction in mesenteric microcirculation.Yakugaku Zasshi. 2010 May;130(5):723-8. doi: 10.1248/yakushi.130.723. Yakugaku Zasshi. 2010. PMID: 20460871 Review.
Cited by
-
Studies of Mg2+/Ca2+ complexes of naturally occurring dinucleotides: potentiometric titrations, NMR, and molecular dynamics.J Biol Inorg Chem. 2012 Aug;17(6):861-79. doi: 10.1007/s00775-012-0903-2. Epub 2012 May 18. J Biol Inorg Chem. 2012. PMID: 22592972
-
International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy.Pharmacol Rev. 2006 Sep;58(3):281-341. doi: 10.1124/pr.58.3.3. Pharmacol Rev. 2006. PMID: 16968944 Free PMC article. Review.
-
The involvement of smooth muscle P2X receptors in the prolonged vasorelaxation response to purine nucleotides in the rat mesenteric arterial bed.Br J Pharmacol. 2002 Apr;135(8):1988-94. doi: 10.1038/sj.bjp.0704663. Br J Pharmacol. 2002. PMID: 11959802 Free PMC article.
-
Uridine adenosine tetraphosphate induces contraction and relaxation in rat aorta.Vascul Pharmacol. 2008 Apr-Jun;48(4-6):202-7. doi: 10.1016/j.vph.2008.03.003. Epub 2008 Mar 28. Vascul Pharmacol. 2008. PMID: 18467183 Free PMC article.
-
Effects of diadenosine polyphosphates on glomerular volume.Br J Pharmacol. 2005 Apr;144(8):1109-17. doi: 10.1038/sj.bjp.0706149. Br J Pharmacol. 2005. PMID: 15711587 Free PMC article.
References
-
- BUSSE R., OGILVIE A., POHL U. Vasomotor activity of diadenosine triphosphate and adenosine tetraphosphate in isolated arteries. Am. J. Physiol. 1988;254:H828–H832. - PubMed
-
- HOYLE C.H.V. Pharmacological activity of adenine dinucleotides in the periphery: possible receptor classes and transmitter function. Gen. Pharmacol. 1990;21:827–831. - PubMed
-
- HOYLE C.H.V., HILDERMAN R.H., PINTOR J.J., SCHLÜTER H., KING B.F. Diadenosine polyphosphates as extracellular signaling molecules. Drug Dev. Res. 2001;52:260–273.
-
- JANKOWSKI J., HAGEMANN J., TEPEL M., VAN DER GIET M., STEPHAN N., HENNING L., GOUNI-BERTHOLD I., SACHINIDIS A., ZIDEK W., SCHLÜTER H. Dinucleotides as growth promoting extracellular mediators: presence of dinucleoside diphosphates Ap2A, Ap2G and Gp2G in releasable granules of platelets. J. Biol. Chem. 2001;276:8904–8909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

