MyoD-positive myoblasts are present in mature fetal organs lacking skeletal muscle
- PMID: 11684706
- PMCID: PMC2150848
- DOI: 10.1083/jcb.200105139
MyoD-positive myoblasts are present in mature fetal organs lacking skeletal muscle
Abstract
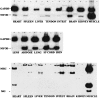
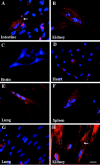
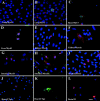
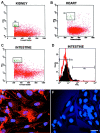
The epiblast of the chick embryo gives rise to the ectoderm, mesoderm, and endoderm during gastrulation. Previous studies revealed that MyoD-positive cells were present throughout the epiblast, suggesting that skeletal muscle precursors would become incorporated into all three germ layers. The focus of the present study was to examine a variety of organs from the chicken fetus for the presence of myogenic cells. RT-PCR and in situ hybridizations demonstrated that MyoD-positive cells were present in the brain, lung, intestine, kidney, spleen, heart, and liver. When these organs were dissociated and placed in culture, a subpopulation of cells differentiated into skeletal muscle. The G8 antibody was used to label those cells that expressed MyoD in vivo and to follow their fate in vitro. Most, if not all, of the muscle that formed in culture arose from cells that expressed MyoD and G8 in vivo. Practically all of the G8-positive cells from the intestine differentiated after purification by FACS. This population of ectopically located cells appears to be distinct from multipotential stem cells and myofibroblasts. They closely resemble quiescent, stably programmed skeletal myoblasts with the capacity to differentiate when placed in a permissive environment.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

