Role of free radicals in the pathogenesis of acute chest syndrome in sickle cell disease
- PMID: 11686897
- PMCID: PMC59517
- DOI: 10.1186/rr70
Role of free radicals in the pathogenesis of acute chest syndrome in sickle cell disease
Abstract
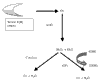
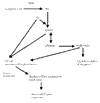
Acute chest syndrome (ACS) of sickle cell disease (SCD) is characterized pathologically by vaso-occlusive processes that result from abnormal interactions between sickle red blood cells (RBCs), white blood cells (WBCs) and/or platelets, and the vascular endothelium. One potential mechanism of vascular damage in ACS is by generation of oxygen-related molecules, such as superoxide (O2-), hydrogen peroxide (H2O2), peroxynitrite (ONOO-), and the hydroxyl (*OH) radical. The present review summarizes the evidence for alterations in oxidant stress during ACS of SCD, and the potential contributions of RBCs, WBCs and the vascular endothelium to this process.
Figures
References
-
- Johnson CS, Verdigem TD. Pulmonary complications of sickle cell disease. Semin Respir Med. 1988;9:287–296.
-
- Gee BE, Platt OS. Sickle erythrocytes adhere to VCAM-1. Blood. 1995;85:268–274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

