Interferon in relapsing-remitting multiple sclerosis
- PMID: 11687131
- PMCID: PMC7017973
- DOI: 10.1002/14651858.CD002002
Interferon in relapsing-remitting multiple sclerosis
Abstract
Background: Recombinant interferons have been shown to suppress both the clinical and magnetic resonance imaging (MRI) measures of disease activity in patients with relapsing remitting multiple sclerosis (RRMS).
Objectives: We performed a Cochrane review of all randomised, placebo-controlled trials of recombinant interferons in RRMS.
Search strategy: Of 208 articles identified by a predefined search strategy, seven of these, reporting randomised trials, met all the selection criteria and form the subject of this review.
Selection criteria: The trials selected were double-blind, placebo-controlled, randomised trials of RRMS patients who were treated with recombinant interferon, given by the subcutaneous or the intramuscular route.
Data collection and analysis: The quality of the trials was variable, with substantial methodological inadequacies in allocation concealment, high proportion and incomplete description of dropouts and failure to adhere to the principles of intention to treat analysis. The baseline characteristics were largely comparable between treatment and placebo groups. Because of prominent treatment-associated side effects, which could be easily identified by patients, these trials could be considered as single blind rather than double-blind.
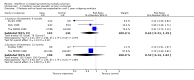
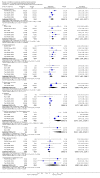
Main results: Although 1215 patients were included in this review, only 919 (76%) contributed to the results concerning exacerbations and progression of the disease at two years. Specifically interferon significantly reduced the occurrence of exacerbations (RR =0.80, 95% CI [0.73,0.88], p<0.001) and progression of the disease (RR =0.69, 95% CI [0.55,0.87], p= 0.002) two years after randomisation. However, the correct assignment of dropouts was essential to the demonstration of efficacy, most conspicuously concerning the effect of the drug on disease progression. If interferon-treated patients who dropped out were deemed to have progressed (worst case scenario) the significance of these effects was lost (RR = 1.31, CI [0.60,2.89], p = 0.5). The evolution in magnetic resonance imaging (MRI) technology in the decade in which these trials were performed and different reporting of data among trials made it impossible to perform a quantitative analysis of the MRI results. Both clinical and laboratory side effects reported in the trials were more frequent in treated patients than in controls. No information was available regarding side effects and adverse events after two years of follow-up. The impact of interferon treatment (and its side effects) on the quality of life of patients was not reported in any trial included in this review.
Reviewer's conclusions: The efficacy of interferon on exacerbations and disease progression in patients with relapsing remitting MS was modest after one and two years of treatment. It was not possible to conduct a quantitative analysis beyond two years. Longer follow-up and more uniform reporting of clinical and MRI outcomes among these trials might have allowed for a more convincing conclusion.
Conflict of interest statement
The review was assembled, analysed and reported independent from any pharmaceutical company input. Prof Rice has participated in clinical trials sponsored by SCHERING, SERONO, BERLEX, BIOGEN, and TEVA. He has received honoraria from these companies. Prof Ebers has received honoraria for lectures found to be sponsored by relevant companies and has been an investigator for several clinical trials in multiple sclerosis including three interferon studies involving SCHERING, SERONO funding. He does not feel his opinions (which are largely critical of the efficacy of interferons) are influenced by these associations. Prof Polman has received honoraria for lecturing, consultancy from companies producing interferon beta. Dr Filippini has received travel expenses from Farmades pharmaceutical company for participating at neurological conferences. Drs Munari, D'Amico, Incorvaia and Parmelli have no conflict of interest.
Figures












Comment in
-
Review: in relapsing-remitting multiple sclerosis, recombinant interferon reduces exacerbations in the first 2 treatment years.ACP J Club. 2002 May-Jun;136(3):104. doi: 10.7326/acpjc-2002-136-3-104. ACP J Club. 2002. PMID: 11985445 No abstract available.
References
References to studies included in this review
Durelli 1994 {published data only}
-
- Bongioanni MR, Durelli L, Ferrero B, Imperiale D, Oggero A, Verdun E, et al. Systemic high‐dose recombinant‐alpha‐2a‐interferon therapy modulates lymphokine production in multiple sclerosis. Journal of the Neurological Sciences 1996;143(1‐2):91‐9. [MEDLINE: ; EMBASE‐1996367643] - PubMed
-
- Durelli L, Bongioanni MR, Cavallo R, Ferrero B, Ferri R, Ferrio MF, et al. Chronic systemic high‐dose recombinant interferon alfa‐2a reduces exacerbation rate, MRI signs of disease activity, and lymphocyte interferon gamma production in relapsing‐remitting multiple sclerosis. Neurology 1994;44(3 Pt 1):406‐13. [MEDLINE: ; EMBASE‐94096622] - PubMed
-
- Durelli L, Bongioanni MR, Ferrero B, Ferri R, Imperiale D, Bradac GB, et al. Interferon alpha‐2a treatment of relapsing‐remitting multiple sclerosis: disease activity resumes after stopping treatment. Neurology 1996;47(1):123‐9. [MEDLINE: ; EMBASE‐1996221919] - PubMed
-
- Durelli L, Bongioanni MR, Ferri R, Cavallo R, Aimo G, Ferrero B, et al. Interferon (IFN)‐Alpha 2a treatment of relapsing‐remitting (R/R) multiple sclerosis (MS): disease activity resumes after stopping treatment [Abstract]. Neurology 1994;44 Suppl (2):A358. - PubMed
IFNB MS Group 1993 {published data only}
-
- Arnason BGW. Interferon beta in multiple sclerosis [Editorial]. Neurology 1993;43(4 Pt 1):641‐3. [EMBASE‐1993121948] - PubMed
-
- Broderick JP, Samaha FJ. Interferon beta treatment of multiple sclerosis [Letter]. Neurology 1994;44(1):186. [MEDLINE: ; EMBASE‐1994036622] - PubMed
-
- Ebers GC, Lublin F, Paty D, Reder A. Treatment of multiple sclerosis with interferon beta‐1b [Letter]. Neurology 1997;49:641‐2. - PubMed
-
- Klapper JA. Interferon beta treatment of multiple sclerosis [Letter]. Neurology 1994;44(1):188. [MEDLINE: ; EMBASE‐1994036622] - PubMed
-
- Longstreth WT Jr, Franklin GM. Interferon beta treatment of multiple sclerosis [Letter]. Neurology 1994;44(1):187. [MEDLINE: ; EMBASE‐1994036622] - PubMed
Knobler 1993 {published data only}
-
- Knobler RL, Greenstein JI, Johnson KP, Lublin FD, Panitch HS, Conway K, et al. Systemic recombinant human interferon‐beta treatment of relapsing‐remitting multiple sclerosis: pilot study analysis and six‐year follow‐up. Journal of Interferon Research 1993;13(5):333‐40. [MEDLINE: ; EMBASE‐94358097] - PubMed
Myhr 1999 {published data only}
-
- Myhr KM, Riise T, Green Lilleas FE, Beiske TG, Celius EG, Edland A, et al. Interferon‐alpha2a reduces MRI disease activity in relapsing‐remitting multiple sclerosis. Norwegian Study Group on Interferon‐alpha in Multiple Sclerosis. Neurology 1999;52(5):1049‐56. [MEDLINE: ; EMBASE‐1999124033] - PubMed
Polman 2003 {published data only}
-
- Polman C, Barkhof F, Kappos L, Pozzilli C, Sandbrink R, Dahlke F, et al. Oral interferon beta‐Ia in relapsing‐remitting multiple sclerosis: a double‐blind randomized study. Multiple Sclerosis 2003;9:342‐48. - PubMed
The MSCRG 1996 {published data only}
-
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. A phase III trial of intramuscular recombinant interferon beta as treatment for exacerbating‐remitting multiple sclerosis: design and conduct of study and baseline characteristics of patients. Multiple Sclerosis Collaborative Research Group (MSCRG). Multiple Sclerosis 1995;1(2):118‐35. [MEDLINE: ] - PubMed
-
- Jacobs LD, Cookfair DL, Rudick RA, Herndon RM, Richert JR, Salazar AM, et al. Intramuscular interferon beta‐1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Annals of Neurology 1996;39(3):285‐94. [MEDLINE: ; EMBASE‐1996105672] - PubMed
-
- Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L, Munschauer III FE, et al. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing‐remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology 1999;53(8):1698‐704. [MEDLINE: ; EMBASE‐1999394535] - PubMed
-
- Rudick RA, Goodkin DE, Jacobs LD, Cookfair DL, Herndon RM, Richert JR, et al. Impact of interferon beta‐1a on neurologic disability in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 1997;49(2):358‐63. [MEDLINE: ; EMBASE‐97265436] - PubMed
-
- Simon JH, Jacobs LD, Campion M, Wende K, Simonian N, Cookfair DL, et al. Magnetic resonance studies of intramuscular interferon beta‐1a for relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group. Annals of Neurology 1998;43(1):79‐87. [MEDLINE: ; EMBASE‐1998090133] - PubMed
The OWIMS 1999 {published data only}
-
- The Once Weekly Interferon for MS Study Group (OWIMS). Evidence of interferon beta‐1a dose response in relapsing‐remitting MS. The OWIMS Study. Neurology 1999;53(4):679‐86. [MEDLINE: ; EMBASE‐1999332822] - PubMed
The PRISMS 1998 {published data only}
-
- Ebers GC. Interferon beta treatment for multiple sclerosis [Letter]. Lancet 1999;353(9151):497. [MEDLINE: ; EMBASE‐1999051720] - PubMed
-
- Li DKB, Paty DW, the UBC MS/MRI Analysis Research Group, the PRISMS Study Group. Magnetic resonance imaging results of the PRISMS trial: a randomized, double‐blind, placebo‐controlled study of interferon‐beta1a in relapsing‐remitting multiple sclerosis. Prevention of Relapses and Disability by Interferon‐beta1a Subcutaneously in Multiple Sclerosis. Annals of Neurology 1999;46(2):197‐206. [MEDLINE: ; EMBASE‐1999285158] - PubMed
-
- The PRISMS (Prevention of Relapses and Disability by Interferon beta‐1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double‐blind placebo‐controlled study of interferon beta‐1a in relapsing/remitting multiple sclerosis. Lancet 1998;352(9139):1498‐504. [MEDLINE: ; EMBASE‐1998379966] - PubMed
References to studies excluded from this review
AUSTIMS 1989 {published data only}
Granger 2003 {published data only}
-
- Granger CV, Wende K, Brownscheidler CM. Use of the FIM instrument in a trial of intramuscular interferon beta‐Ia for disease progression in relapsing‐remitting multiple sclerosis. American Journal of Phisical Medicine & Rehabilitation 2003;82(6):427‐36. - PubMed
Herndon 1999 {published data only}
-
- Herndon RM, Jacobs LD, Coats ME, Goodkin DE, Mass MK, Richert JR, et al. Results of an ongoing, open‐label, safety‐extension study of interferon beta‐1a (Avonex) treatment in multiple sclerosis. International Journal of MS Care 1999;1(2):1‐6.
Hirsch 1986 {published data only}
-
- Hirsch RL, Johnson KP. The effects of long‐term administration of recombinant alfa‐2 interferon on lymphpcyte substes, proliferation, and suppressor cell function in multiple sclerosis. Journal of Interferon Research 1986;6(2):171‐7. [MEDLINE: ] - PubMed
Jacobs 2000 {published data only}
-
- Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ. Intramuscular interferon beta‐1a therapy initiated during a first demyelinating event in multiple sclerosis. New England Journal of Medicine 2000;343(13):898‐904. - PubMed
Knobler 1984 {published data only}
-
- Knobler RL, Panitch HS, Braheny SL, Sipe JC, Rice GP, Huddlestone JR, et al. Clinical trial of natural alpha interferon in multiple sclerosis. Annals of the New York Academy of Sciences 1984;436:382‐8. - PubMed
Milanese 1990 {published data only}
Pozzilli 1997 {published data only}
-
- Koudriavtseva T, Pozzilli C, Fiorelli M, Gasperini C, Bagnato F, Galgani S, et al. Determinants of Gd‐enhanced MRI response to IFN‐beta‐1a treatment in relapsing‐remitting multiple sclerosis. Multiple Sclerosis 1998;4(5):403‐7. [MEDLINE: ; EMBASE‐1998381761] - PubMed
-
- Paolillo A, Bastianello S, Frontoni M, Gasperini C, Giugni E, Ciccarelli O, et al. Magnetic resonance imaging outcome of new enhancing lesions in relapsing‐remitting multiple sclerosis patients treated with interferon beta‐1a. Journal of Neurology 1999;246(6):443‐8. [MEDLINE: ; EMBASE‐1999244760] - PubMed
-
- Pozzilli C, Bastianello S, Koudriatseva T, Gasperini C, Mainero C, Giugni E, et al. An open randomised trial with two different doses of recombinant interferon beta‐1a in relapsing‐remitting multiple sclerosis: clinical and MRI results at 24 months [Abstract]. Journal of Neurology 1997;244:S25.
-
- Pozzilli C, Bastianello S, Koudriavtseva T, Gasperini C, Bozzao A, Millefiorini E, et al. Magnetic resonance imaging changes with recombinant human interferon‐beta‐1a: a short term study in relapsing‐remitting multiple sclerosis. Journal of Neurology, Neurosurgery & Psychiatry 1996;61(3):251‐8. [MEDLINE: ; EMBASE‐1996271523] - PMC - PubMed
Rudge 1995 {published data only}
-
- Rudge P, Miller D, Crimlisk H, Thorpe J. Does interferon beta cause initial exacerbation of multiple sclerosis? [Letter]. Lancet 1995;345(8949):580. [MEDLINE: ; EMBASE‐1995077181] - PubMed
Rudick 1998 {published data only}
-
- Rudick RA, Simonian NA, Alam JA, Campion M, Scaramucci JO, Jones W, et al. Incidence and significance of neutralizing antibodies to interferon beta‐1a in multiple sclerosis. Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 1998;50(5):1266‐72. [MEDLINE: ; EMBASE‐1998175405] - PubMed
Schwartz 1997 {published data only}
-
- Schwartz CE, Coulthard‐Morris L, Cole B, Vollmer T. The quality‐of‐life effects of interferon beta‐1b in multiple sclerosis. An extended Q‐TWIST analysis. Archives of Neurology 1997;54(12):1475‐80. [MEDLINE: ; EMBASE‐1997379054] - PubMed
Simon 1999 {published data only}
-
- Simon JH, Jacobs LD, Campion MK, Rudick RA, Cookfair DL, Herndon RM, et al. A longitudinal study of brain atrophy in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 1999;53(1):139‐48. [MEDLINE: ; EMBASE‐1999247274] - PubMed
References to studies awaiting assessment
Abdul‐Ahad 1997 {published data only}
-
- Abdul‐Ahad AK, Galazka AR, Revel M, Biffoni M, Borden ED. Incidence of antibodies to interferon‐beta in patients treated with recombinant human interferon‐beta1a from mammalian cells. Cytokines Cellular & Molecular Therapy 1997;3(1):27‐32. [MEDLINE: ; EMBASE‐1997288646] - PubMed
Camenga 1986 {published data only}
-
- Camenga DL, Johnson KP, Alter M, Engelhardt CD, Fishman PS, Greenstein JI, et al. Systemic recombinant alfa‐2 interferon therapy in relapsing multiple sclerosis. Archives of Neurology 1986;43(12):1239‐46. [MEDLINE: ] - PubMed
Edwards 1998 {published data only}
-
- Edwars S, Zvartau M, Clarke H, Irving W, Blumhardt LD. Clinical relapses and disease activity on magnetic resonance imaging associated with viral upper respiratory tract infections in multiple sclerosis. Journal of Neurology Neurosurgery & Psychiatry 1998;64(4):736‐41. [MEDLINE: ; EMBASE‐1998198807] - PMC - PubMed
Additional references
Barnes 1997
-
- Barnes D, Hughes RA, Morris RW, Wade‐Jones O, Brown P, Britton T, et al. Randomised trial of oral and intravenous methylprednisolone in acute relapses of multiple sclerosis. Lancet 1997;349(9056):902‐6. [MEDLINE: ; EMBASE‐1997097394] - PubMed
Clarke 2000
-
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook [updated July 1999]; Section 6.3. In: The Cochrane Library [database on CDROM]. The Cochrane Collaboration. Oxford: Update Software; 2000.
Cohen 2001
-
- Cohen JA, Cutter GR, Fischer JS, Goodman AD, Heidenreich FR, Jak AJ, et al. Use of the multiple sclerosis functional composite as an outcome measure in a phase 3 clinical trial. Archives of Neurology 2001;58(6):961‐7. [MEDLINE: ] - PubMed
Confavreux 2000
-
- Confavreux C, Vukusic S, Moreau T, Adeleine P. Relapses and Progression of Disability in Multiple Sclerosis. New England Journal of Medicine 2000;343(20):1430‐8. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
European Group 1998
-
- European Study Group on Interferon beta‐1b in Secondary Progressive MS. Placebo‐controlled multicentre randomized trial of interferon beta‐1b in treatment of secondary progressive multiple sclerosis. Lancet 1998;52:491‐7. - PubMed
Goodkin 1992
-
- Goodkin DE, Cookfair D, Wende K, Bourdette D, Pullicino P, Scherokman B, et al. Inter‐ and intrarater scoring agreement using grades 1.0 to 3.5 of the Kurtzke Expanded Disability Status Scale (EDSS). Multiple Sclerosis Collaborative Research Group. Neurology 42;4:859‐63. [MEDLINE: ] - PubMed
Goodkin 2000
-
- Goodkin DE and the North American Study Group on Interferon beta‐1b in Secondary Progressive MS. Interferon beta‐1b in secondary progressive MS: clinical and MRI results of a 3‐year randomized controlled trial [Abstract]. American Academy of Neurology 2000.
Hauser 1983
-
- Hauser SL, Dawson DM, Lehrich JR, Beal MF, Kevy SV, Propper RD, et al. Intensive immunosuppression in progressive multiple sclerosis. A randomized, three‐arm study of high‐dose intravenous cyclophosphamide, plasma exchange, and ACTH. New England Journal of Medicine 1983;308(4):173‐80. [MEDLINE: ] - PubMed
Hohlfeld 1997
-
- Hohlfeld R. Biotechnological agents for the immunotherapy of multiple sclerosis. Principles, problems and perspectives [Review]. Brain 1997;120(Pt 5):865‐916. [MEDLINE: ; EMBASE‐1997157798] - PubMed
Hughes 2000
-
- Hughes RAC and the SPECTRIMS group. Relapsing versus nonrelapsing SPMS: different prognosis and response to interferon therapy in the SPECTRIMS study. Neurology 2000;54 Suppl (3):A233.
Jacobs 1981
-
- Jacobs L, O'Malley J, Freeman A, Ekes R. Intrathecal interferon reduces exacerbations of multiple sclerosis. Science 1981;214(4524):1026‐8. [MEDLINE: ] - PubMed
Knobler 1984a
-
- Knobler RL, Panitch HS, Braheny SL, Sipe JC, Rice GP, Huddlestone JR, et al. Systemic alpha‐interferon therapy of multiple sclerosis. Neurology 1984;34(10):1273‐9. [MEDLINE: ] - PubMed
Kurtzke 1961
-
- Kurtzke JF. On the evaluation of disability in multiple sclerosis. Neurology 1961;11:686‐94. - PubMed
Kurtzke 1983
-
- Kurtzke JF. Rating neurological impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33(11):1444‐52. [MEDLINE: ] - PubMed
Liu 2000
-
- Liu C, Blumhardt LD. Disability outcome measures in therapeutic trials of relapsing‐remitting multiple sclerosis: effects of heterogeneity of disease course in placebo cohorts. Journal of Neurology, Neurosurgery & Psychiatry 2000;68(4):450‐7. [MEDLINE: ; EMBASE‐2000163002 EMBASE‐2000163002] - PMC - PubMed
Noseworthy 1999
-
- Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis [Review]. Nature 1999;399(6738 Suppl):40‐7. [MEDLINE: ; EMBASE‐1999223163] - PubMed
Poser 1983
-
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Annals of Neurology 1983;13(3):227‐31. [MEDLINE: ] - PubMed
Rice 1998
-
- Rice G, Ebers G. Interferons in the treatment of multiple sclerosis: do they prevent the progression of the disease?. Archives of Neurology 1998;55(12):1578‐80. [MEDLINE: ] - PubMed
Schumacher 1968
-
- Schumacher GA, Beebe G, Kilber RF, et al. Problems of experimental trials of therapy in MS: report of the panel on evaluation of experimental trials in MS. Annals of the New York Academy of Sciences 1968;122:552‐68. - PubMed
Smeeth 1999
vanBoxel‐Dezaire2000
-
- Boxel‐Dezaire AH, Trigt‐Hoff SC, Killestein J, Schrijver HM, Houwelingen JC, Polman CH, et al. Contrasting responses to interferon beta‐1b treatment in relapsing‐remitting multiple sclerosis: does baseline interleukin‐12p35 messenger RNA predict the efficacy of treatment ?. Annals of Neurology 2000;48(3):313‐22. [MEDLINE: ; EMBASE‐2000315459] - PubMed
Weinshenker 1989
-
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, et al. The natural history of multiple sclerosis: a geographically based study. 2. Predictive value of the early clinical course. Brain 1989;112 (Pt 6):1419‐28. [MEDLINE: ; EMBASE‐1990024556] - PubMed
Yusuf 1985
-
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

